Kit for detecting 10 respiratory tract infection pathogens and application method thereof
A respiratory and pathogenic technology, applied in the field of molecular biology, can solve problems such as delayed treatment timing, inability to culture bacteria, poor culture effect, etc., and achieve the effect of reducing pollution interference, reducing the possibility of pollution and result deviation, and avoiding sample contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kit for detecting 10 respiratory infection pathogens, the kit consists of primer-probe mixture, RT-PCR buffer, mixed enzyme solution, positive quality control and RNase-free water; RT-PCR buffer includes PCR buffer, dATP , dUTP, dCTP, dGTP and MgCl 2 ; The mixed enzyme solution includes HotStart Taq enzyme, reverse transcriptase and UNG enzyme; the positive quality control contains plasmids corresponding to the amplified gene sequences of the above 10 respiratory pathogens.
[0054] The final concentration of the primer in the amplification system is 100-1000nM; the final concentration of the probe in the amplification system is 50-500nM.
[0055] The reaction system of the kit is 25 μL, specifically: 2 μL of nucleic acid template, 20 μL of RT-PCR buffer, 1 μL of mixed enzyme solution, and 2 μL of primer-probe mixture.
[0056] Kit operation and result determination
[0057] (1) Add 2 μL each of sample DNA / RNA co-extraction template (extracted from human sputum, thr...
Embodiment 2
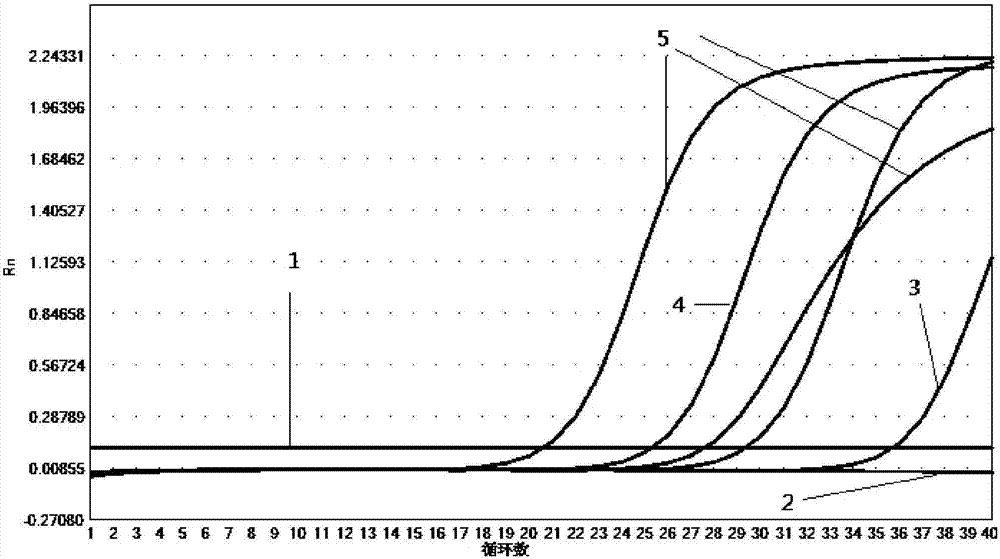
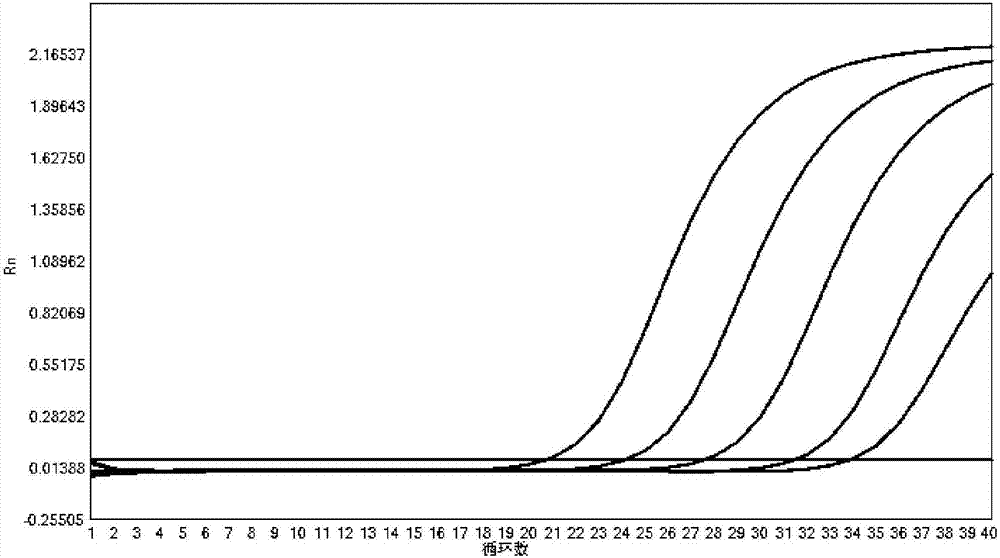
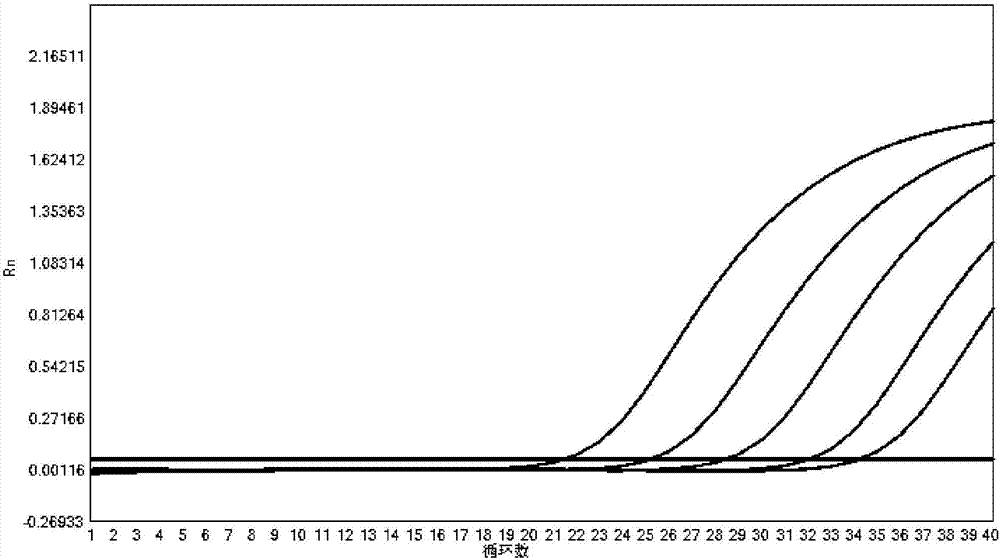
[0068] Use this kit to detect the corresponding pathogen plasmid samples respectively, according to the dilution concentration of 10 5 copies / μL, 10 4 copies / μL, 10 3 copies / μL, 10 2 Sensitivity test was carried out with five gradients of copies / μL and 10copies / μL. Detection was carried out in the same manner as in Example 1.
[0069] The detection result shows that the sensitivity of the kit of the present invention is good, and the Ct value changes in a gradient with concentration reduction, the results are shown in Figure 2-11 .
[0070] The test results show that the kit is effective in the diagnosis of respiratory pathogens Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, Bordetella pertussis, rhinovirus, influenza A virus, influenza B virus, respiratory adenovirus, syncytial virus, parainfluenza virus High sensitivity, the detection sensitivity of each item can reach 100copies / μL.
Embodiment 3
[0072] Use this kit to detect Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, Bordetella pertussis, rhinovirus, influenza A virus, influenza B virus, respiratory adenovirus, syncytial virus, parainfluenza virus and other samples. Detection was carried out in the same manner as in Example 1.
[0073] The detection result shows that the kit of the present invention can only detect the pathogen corresponding to the detection reagent, and will not detect other pathogens.
[0074] The test results show that the kit is specific for the detection of respiratory pathogens Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, Bordetella pertussis, rhinovirus, influenza A virus, influenza B virus, respiratory adenovirus, syncytial virus, parainfluenza virus Strong, does not cross-react with nucleic acids of other pathogens, see results Figure 12-21 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com