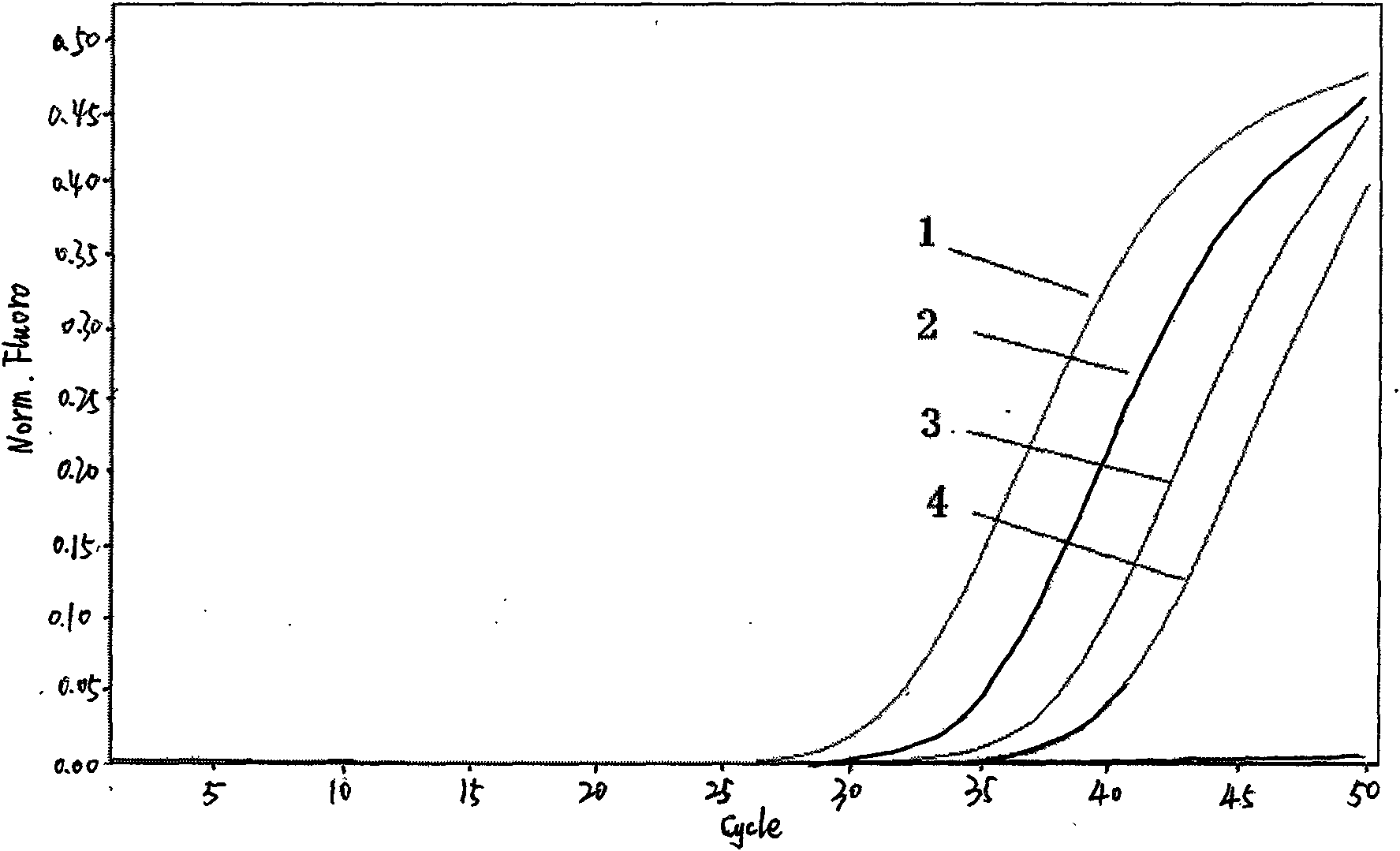
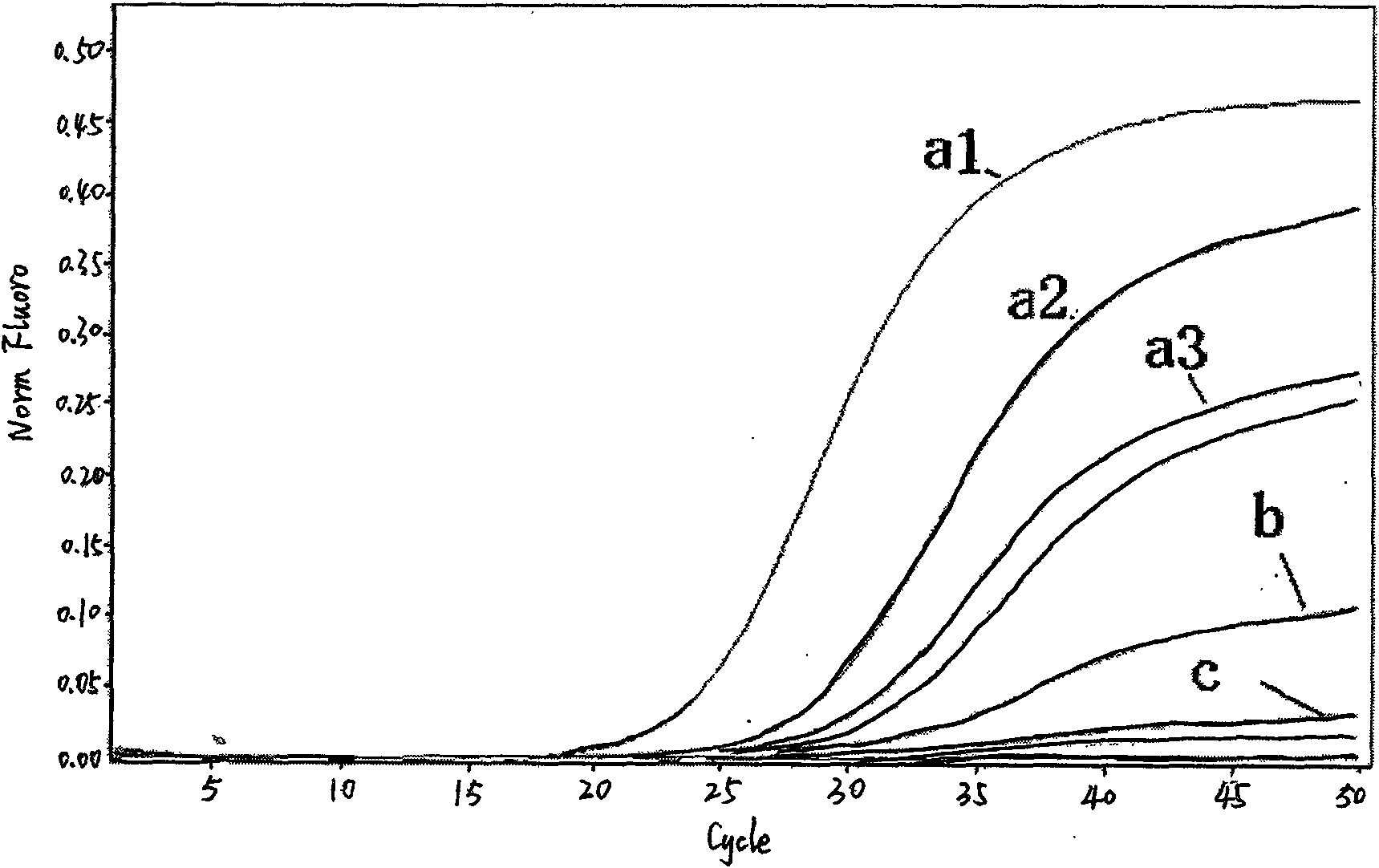
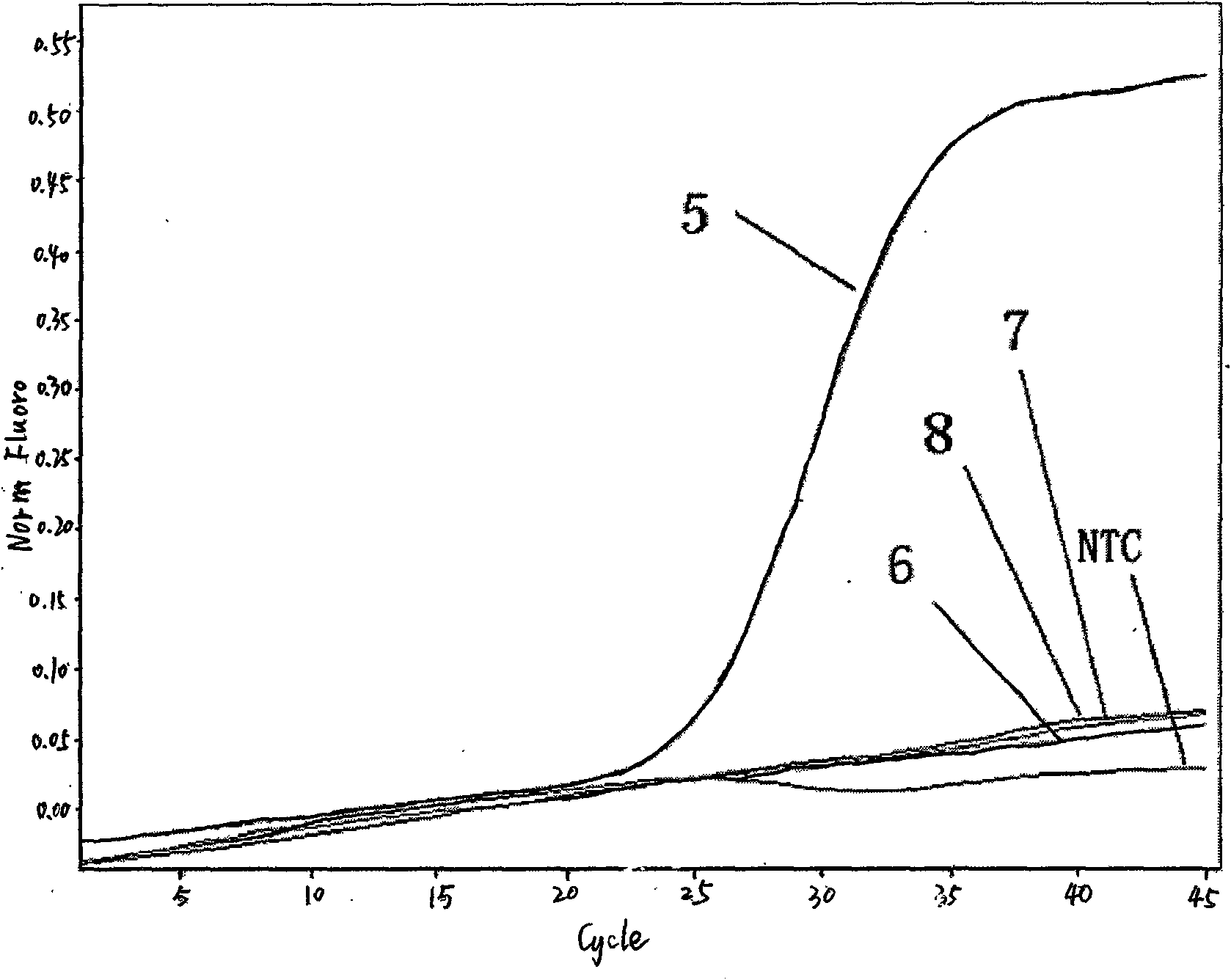
Fluorescent quantitative detection kit of H1N1 influenza virus A and detection method thereof
A technology for influenza virus and fluorescence quantification, applied in the direction of fluorescence/phosphorescence, microbiological-based methods, biochemical equipment and methods, etc., can solve the problems of increasing biosafety risks by detecting positive references, and achieve highly specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1 Materials and methods
[0046] Virus strains and clinical specimens:
[0047] Influenza A H1N1, SWH1N1, seasonal H1N1, and seasonal H3N1 virus strains were isolated from Hong Kong and Zhejiang Center for Disease Control and Prevention. The clinical samples were obtained from the throat swabs of suspected patients with the recent outbreak of H1N1 virus in Zhejiang Province. The samples were collected and transported to the laboratory with ice.
[0048] 1.2 Primers and probes
[0049] A(H1N1), seasonal H1N1, porcine H1N1 influenza virus strains from all over the world were downloaded from the NCBI gene bank in the United States. Homology comparisons were carried out, and influenza A H1N1-specific primers and Taqman probes were designed in the HA gene region of all influenza virus genomes. The sequences are as follows:
[0050] Upstream primer Type A H1N1-FP: 5'-AGGTTTGAGATATTCCCCAAGACA-3'
[0051] Downstream Primer Type A H1N1-RP: 5'-AATTTTTGTAGAAGCTTTTTGCTCC-3'
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com