Typing detection kit for influenza virus
A technology of influenza virus and influenza A virus, which is applied in the field of influenza virus typing detection kits, can solve problems such as antibody-specific interference, and achieve the effects of reducing pollution, improving detection efficiency, and high throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
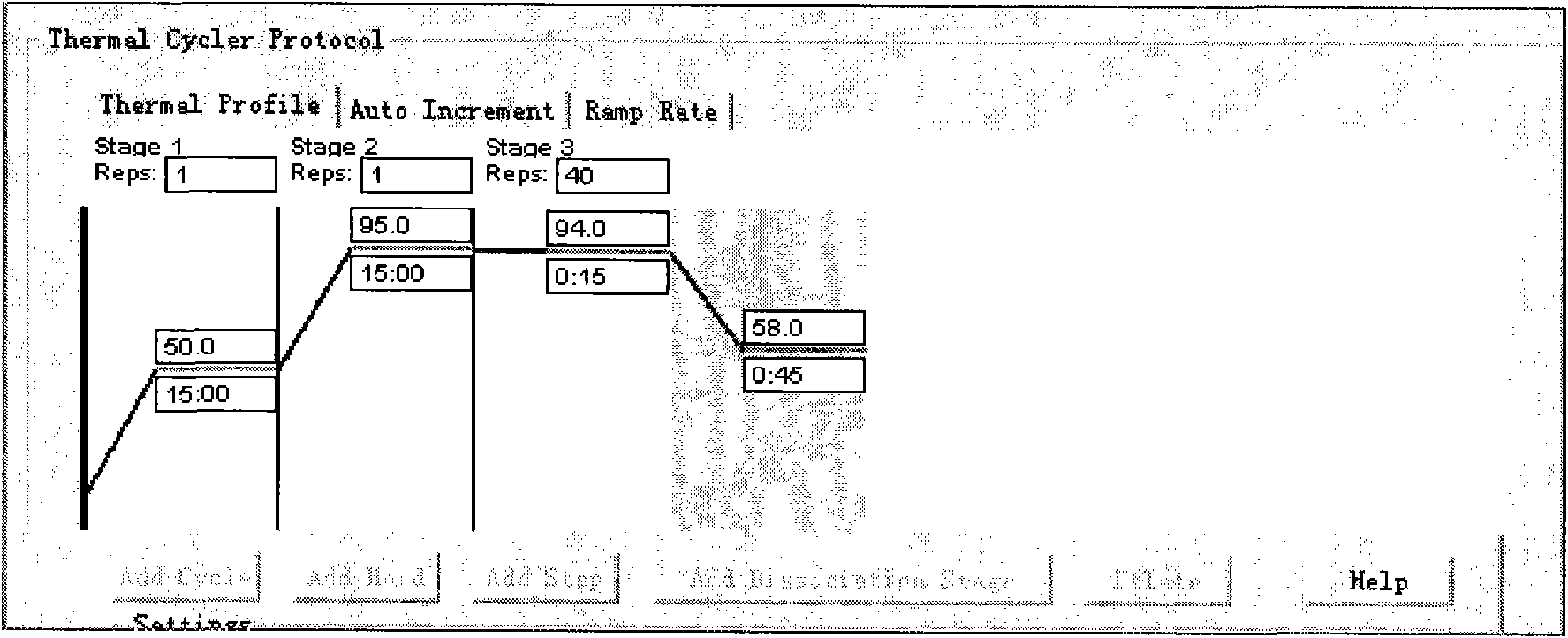
[0027] Example 1 Influenza virus typing detection kit and its use
[0028] 1. Prepare a kit including the following components: 1 tube of primer-probe mixture (25 μl / tube), 1 tube of RT-PCR reaction solution (250 μl / tube), 1 tube of RT-PCR reaction enzyme system (75 μl / tube), positive Quality control (200μl / tube) 1 tube, negative quality control (200μl / tube) 1 tube, DEPC H 2 O (2000μl / tube) 1 tube.
[0029] 2. Collection, preservation and transportation of specimens
[0030] 2.1 Specimen types: nasal swabs, throat swabs, nasopharyngeal aspirates and other respiratory samples.
[0031] 2.2 Specimen collection, storage and transportation: Use sterilized cotton swabs to take nasal or throat secretions, and immediately put them into a small test tube containing 2 mL of sterilized normal saline or Eagle solution or 0.5% hydrolyzed milk protein Hanks solution, and label it , put it in a refrigerator and carry it to the laboratory or freeze it at low temperature (-20℃~-70℃). Dry ...
Embodiment 2
[0041] Example 2 Application of Influenza Typing Detection Kit to Detection of Clinical Samples
[0042] Select 3 cases identified as influenza virus negative through the virus culture method, and 3 cases identified as influenza virus positive specimens through the virus culture method, the nucleic acid extraction of the specimen, PCR amplification and result analysis steps are carried out with reference to Example 1, and negative and positive are carried out at the same time Testing of quality control products.
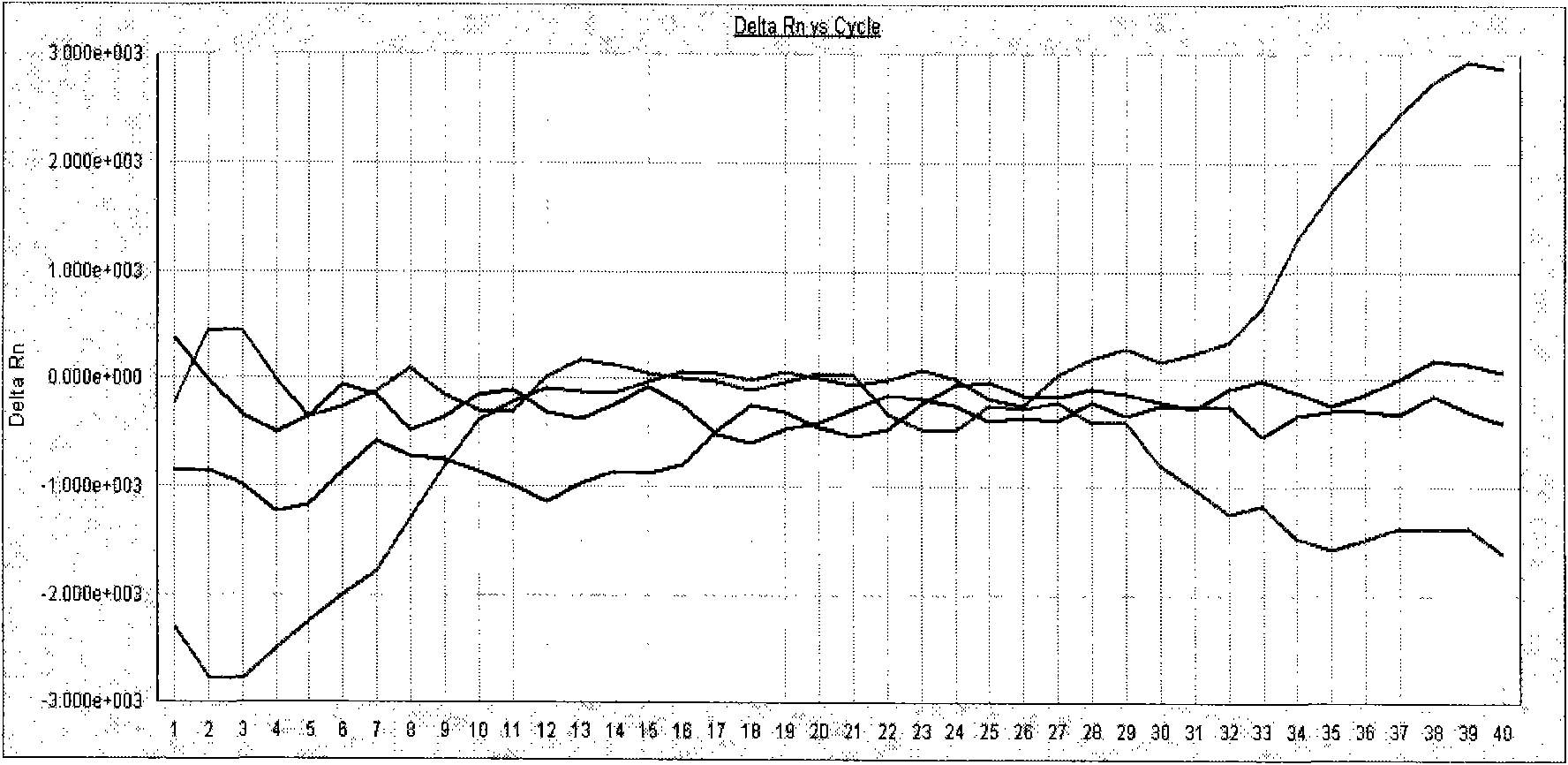
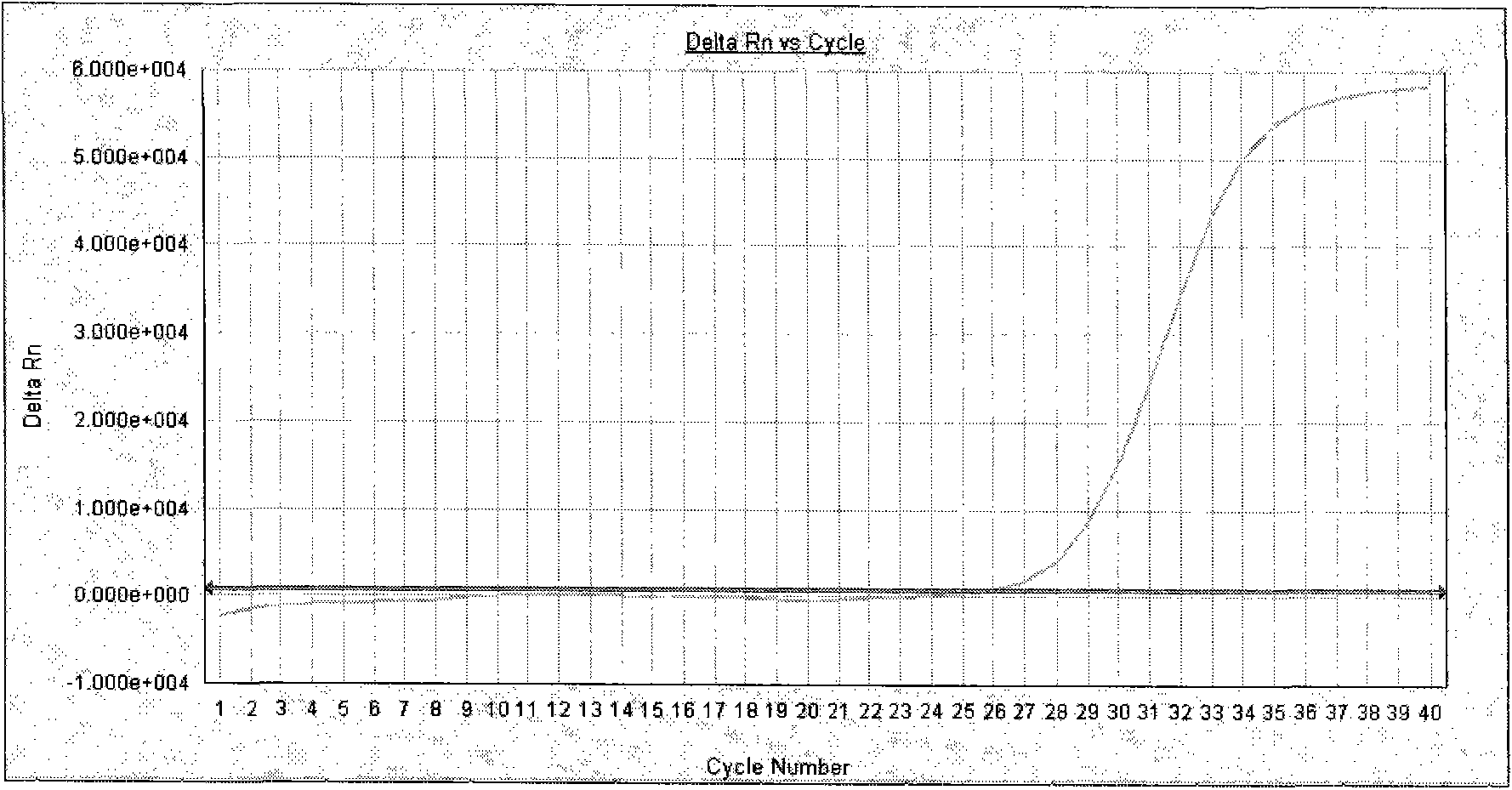
[0043] Test result: the amplification curve of the negative quality control product is not S-shaped (see attached figure 2 ), the amplification curve of the positive quality control product is an obvious S-shaped curve (see attached image 3 , 4, 5), both negative and positive quality control products meet the quality control requirements of the kit, so the test results of the samples to be tested are valid. From the detection results of the specimens to be tested...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com