Preparation and application of benzoyl hydrazine derivative
A technology of benzohydrazide and derivatives, which is applied in the preparation and application field of benzohydrazide derivatives, can solve the problems of quenching response signal, inconvenient detection of metal ions, and large interference of coexisting ions, etc., and achieves easy purification , low cost and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
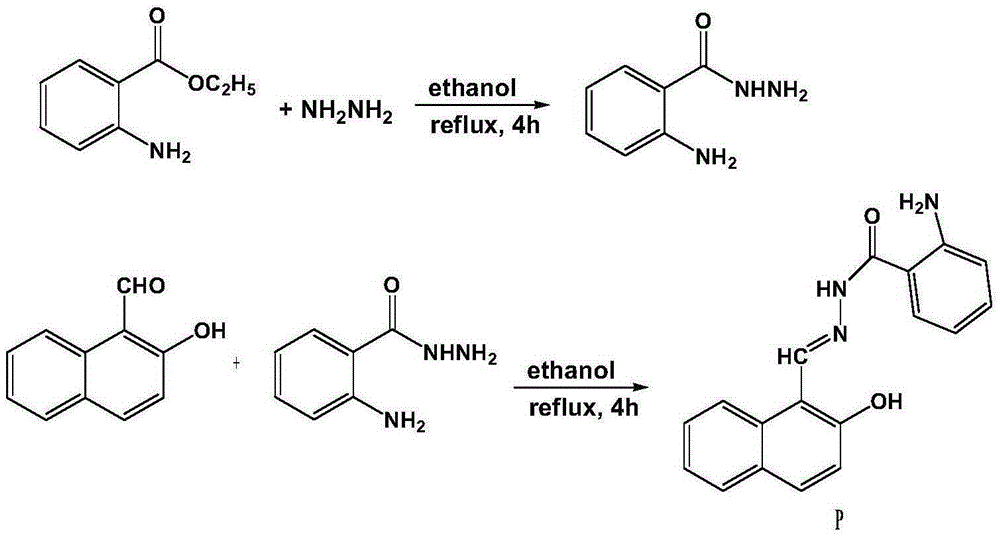
[0022] The synthesis of benzohydrazide derivative P compound shown in formula 1 (see figure 1 ):
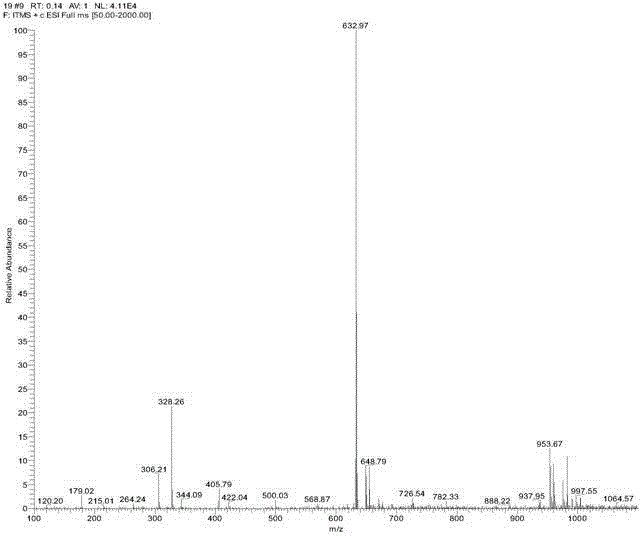
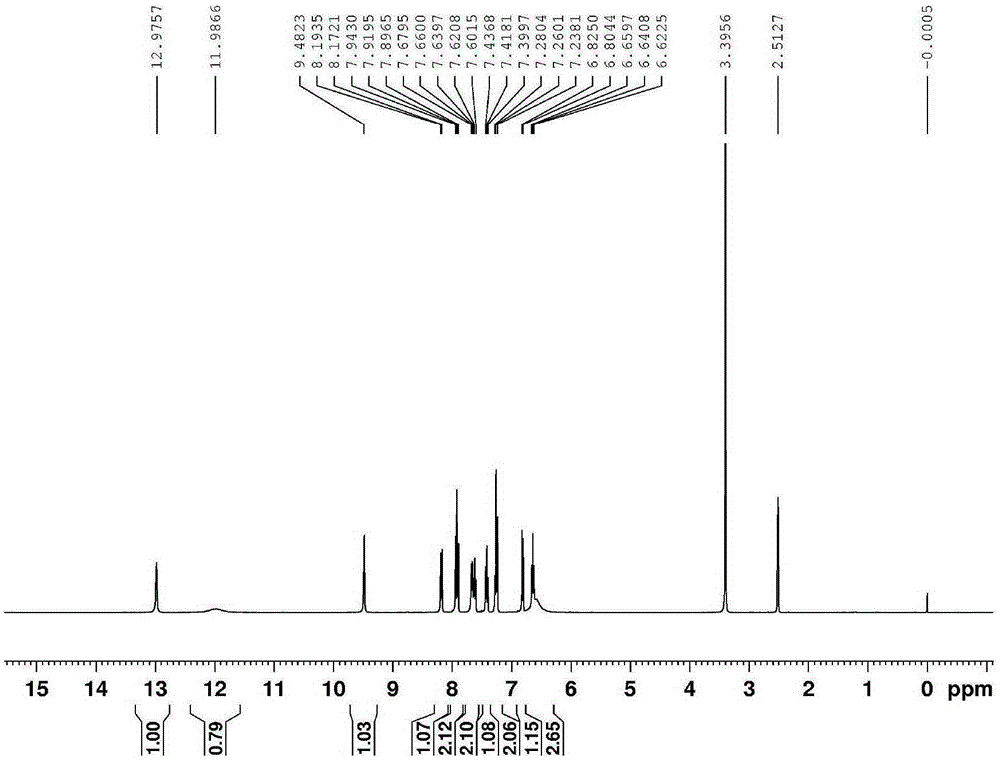
[0023] Heat 1 mmol of ethyl anthranilate and 1.5 mmol of hydrazine hydrate in absolute ethanol to reflux for 4 h. After cooling to room temperature, the precipitated solid is filtered, washed with absolute ethanol, and the intermediate compound obtained after drying is directly used in the next step. The intermediate was reacted with o-hydroxynaphthaldehyde at a molar ratio of 1:1 in absolute ethanol for 4 hours at 80°C. After cooling, the precipitated solid was filtered with suction, washed with a large amount of water and absolute ethanol, and dried to obtain a light yellow solid pure Product P (see figure 2 and 3 ), 85% yield.
[0024] From figure 2 Molecular ion peaks and image 3 The chemical shift of H in can be used to know the formation of benzoylhydrazide derivative P.
[0025] Optical Recognition of Mg2+ by Benzohydrazide Derivative P
[0026] 1) The diluent is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com