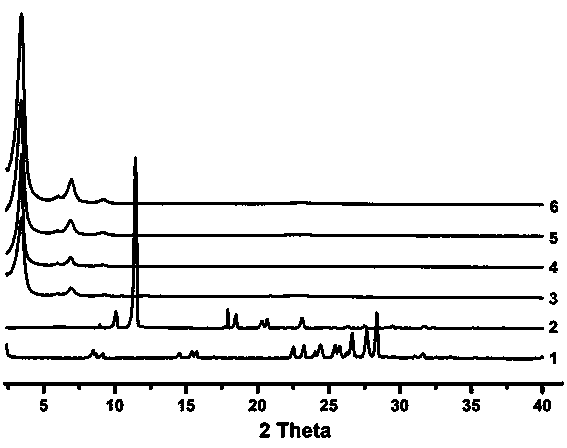
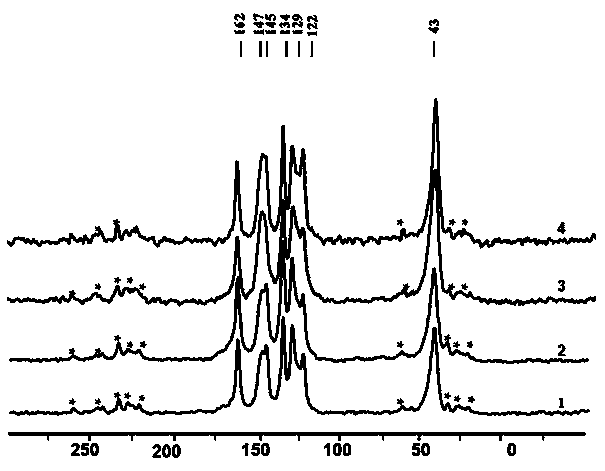
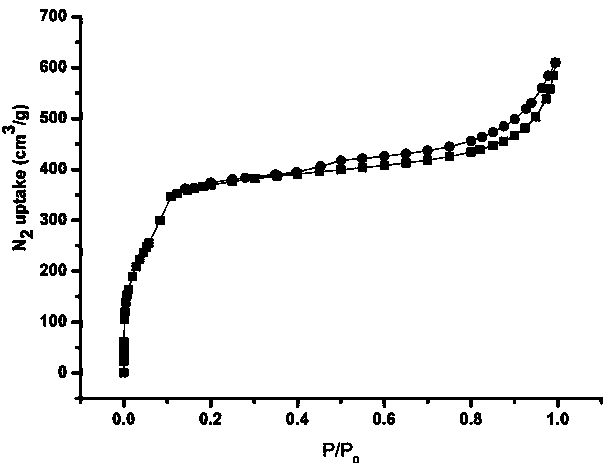
Synthetic method and application of covalent organic framework (COF) material
A technology of covalent organic framework and synthesis method, which is applied in the field of synthesis of covalent organic framework materials, and can solve problems such as COF materials that have not been seen yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The starting materials trimesaldehyde (20 mg, 0.123 mmol) and 2,5-bis(N,N-dimethyl)amino-1,4-benzenedihydrazide (52 mg, 0.186 mmol, compound 2) were added to In a pressure-resistant tube (volume: 20 mL, tube height 18 cm, tube length 9 cm), add 0.5 mL of ethanol and 1.5 mL of mesitylene, shake well, add 0.4 mL of 3 mol / L acetic acid, and dissolve the system with liquid Vacuumize after freezing with nitrogen, seal the pipe diameter with fire, put it in an oven, and react at 120°C for 3 days. After the reaction, the diameter of the tube was knocked out, acetone was added, the volume was replaced with acetone, and the tube was allowed to stand for 12 h, centrifuged and dried naturally. 50 mg of COF-LZU5 was obtained with a yield of 70%.
Embodiment 2
[0048] Add the raw materials trimesaldehyde (50 mg, 0.308 mmol) and 2,5-bis(N,N-dimethyl)amino-1,4-benzenedihydrazide (130 mg, 0.465 mmol) into the pressure tube medium (volume: 20 mL, tube height 18 cm, tube length 9 cm), add 1.25 mL of dioxane, 3.75 mL of mesitylene, shake well, add 1.0 mL of 3 mol / L acetic acid, and drain the system Vacuumize after freezing with nitrogen, seal the pipe diameter with fire, put it in an oven, and react at 120°C for 3 days. After the reaction, the diameter of the tube was knocked out, acetone was added, the volume was replaced with acetone, and the tube was allowed to stand for 12 h, centrifuged and dried naturally. 130 mg of COF-LZU5 was obtained with a yield of 72%.
Embodiment 3
[0050] Add the starting materials trimesaldehyde (50 mg, 0.308 mmol) and 2,5-bis(N,N-dimethyl)amino-1,4-benzenedihydrazide (130 mg, 0.465 mmol) to a 25 mL round In the bottom flask, add 1.25 mL solvent dioxane and 3.75 mL mesitylene, shake well, add 1.0 mL 3 mol / L acetic acid, stir and reflux for 3 days. After the reaction, acetone was added, washed three times with acetone, centrifuged and dried naturally to obtain COF-LZU5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com