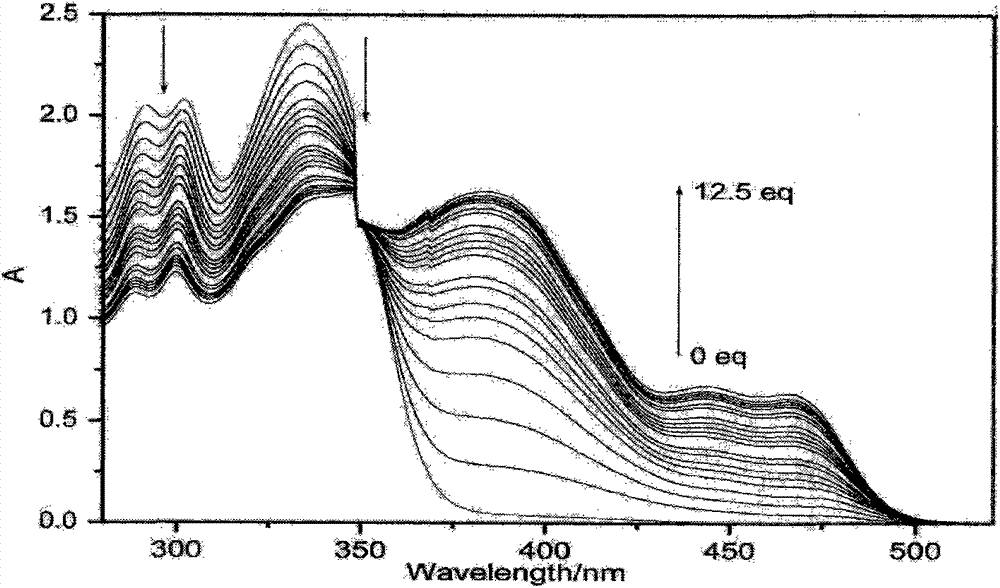
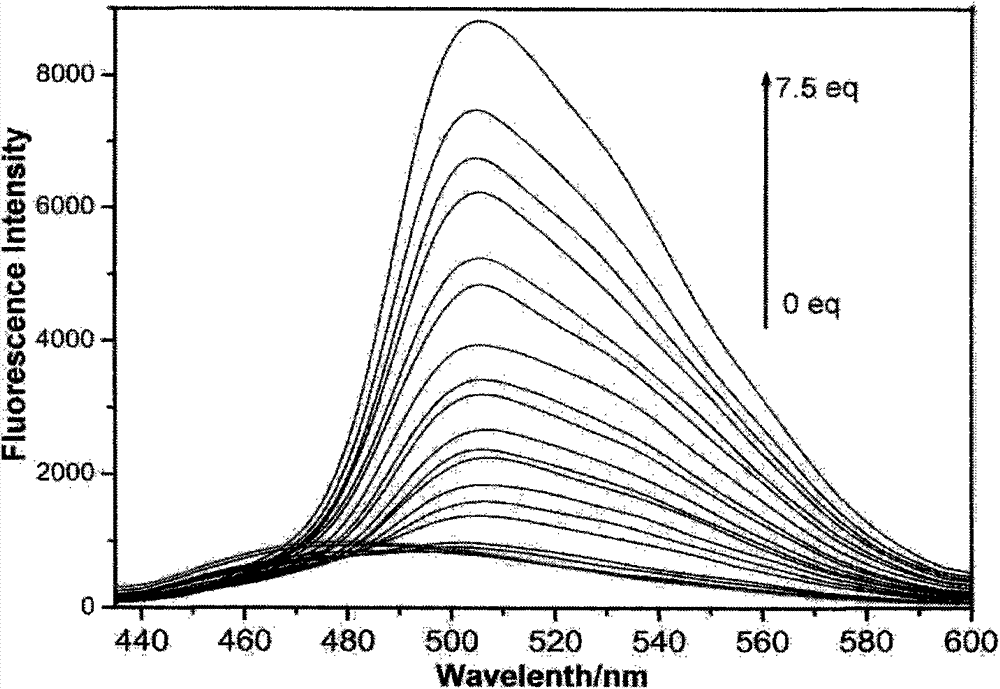
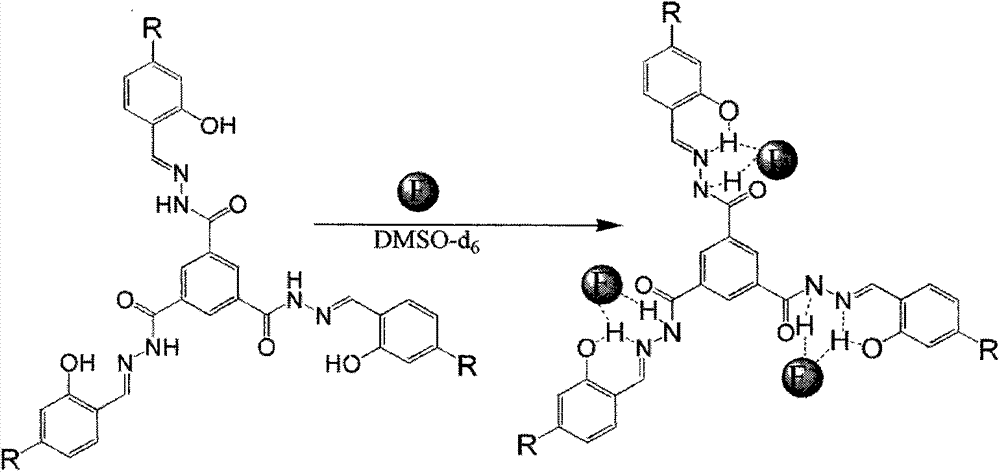
Preparation method of trimesoyl hydrazone series derivatives and application of trimesoyl hydrazone series derivatives as probe molecules for identifying fluorine ions
A technology of trimesoyl hydrazone and trimesoyl hydrazone, applied in the field of trimesoyl hydrazone derivatives and preparation thereof, can solve problems such as few studies, and achieve the effects of simple process, high yield and convenient recycling and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The trimesoylhydrazone derivative has a molecular structure as shown in the following formula:
[0033]
[0034] Wherein R=H, which is a trimesoylhydrazone derivative a;
[0035] R=OH, is trimesoylhydrazone derivative b;
[0036]R=CH 3 O, is a trimesoylhydrazone derivative c.
[0037] The recognition receptor of the trimesoylhydrazone derivative of the present invention has been determined by elemental analysis and hydrogen nuclear magnetic resonance spectrum, and the results are as follows:
[0038] Trimellitichydrazone derivative a: white microcrystals, yield 91.15%, 1 H NMR (d 6 -DMSO): δ H 6.96 (t, 6H), 7.34 (t, 3H), 7.64 (d, 3H), 8.74 (t, 6H), 11.18 (s, 3H), 12.47 (s, 3H). Elemental analysis calcd for C 30 h 24 o 6 N 6 : C63.82%, H4.28%, O17.00%; Found: C63.56%, H4.18%, O17.09%.
[0039] Trimellitichydrazone derivative b: white microcrystals, yield 92.21%, 1 HNMR(d 6 -DMSO): δ H 6.35 (m, 6H), 7.34 (d, 3H), 8.60 (d, 6H), 9.97 (s, 3H), 11.33 (s, 3H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com