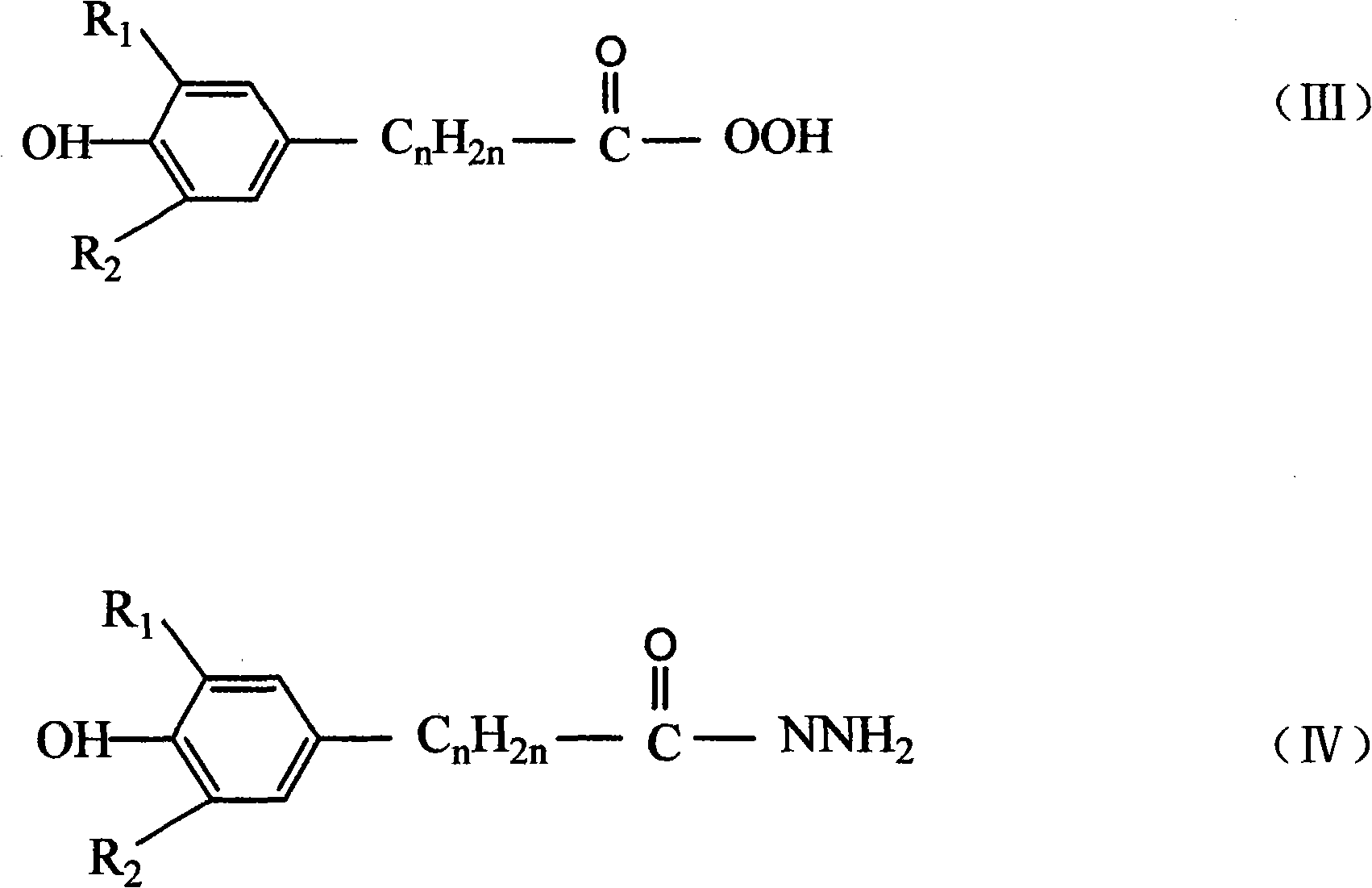
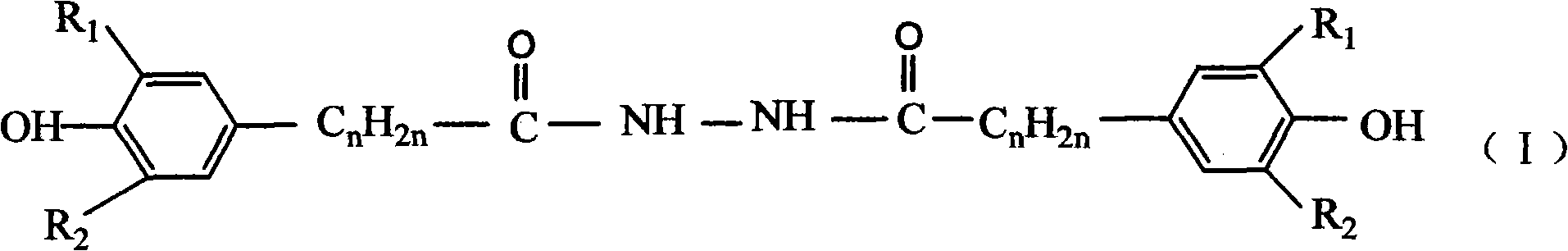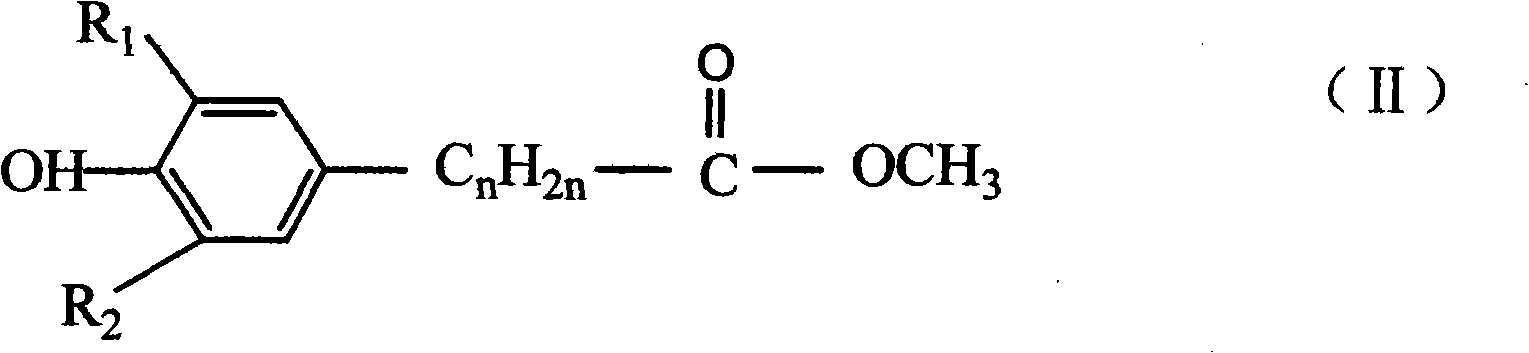Catalytic preparation method for hindered phenol derivative antioxygen
A technology for the preparation of hindered phenols by catalysis, which is applied in the preparation of hydrazides, organic chemistry, etc., can solve the problems of unfavorable promotion and use, high product cost, and difficulty in recycling, so as to improve the utilization rate of reaction raw materials, reduce reaction costs, and shorten the reaction time. the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] As mentioned above, the catalytic preparation method of the hindered phenolic derivative antioxidant disclosed by the present invention comprises the following steps:
[0042] The first step, acidification reaction:
[0043] in N 2Under protection, add an ester compound with a hindered phenol structure and a first solvent into a reactor, the molar ratio of the first solvent to the ester compound with a hindered phenol structure is 5-20:1; the first solvent At least one selected from the following: methanol, ethanol, aqueous methanol, aqueous ethanol; for example: the molar ratio of the first solvent to the ester compound with a hindered phenol structure can be 5:1, 6:1, 7:1, 8:1, 9:1, 10:1, 11:1, 12:1, 13:1, 14:1, 15:1, 16:1, 17:1, 18:1, 19:1 or 20:1 1. When using any two, more than two, or all of methanol, ethanol, methanol aqueous solution, and ethanol aqueous solution, the ratio is arbitrary;
[0044] The reaction temperature of the acidification reaction is contr...
Embodiment 1
[0061] Add 1mol of methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (that is, an ester compound with a hindered phenol structure) and 500ml of methanol into a 1L four-necked flask, pass N 2 , start stirring, and heat in a water bath. When the temperature is constant at 60° C., start to add 220 ml of 30% NaOH solution dropwise. After the dropwise addition, slowly heat to 65°C to make the reaction proceed smoothly. After reacting for 4 hours, transfer it to a beaker, then add 1600mL of 2N dilute hydrochloric acid to neutralize, and a white precipitate precipitates, stir for 2 hours, filter, wash with water until neutral, and dry to obtain 290g of intermediate acid.
[0062] Add 1 mol of methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate and 2000ml of methanol into a 5L three-necked flask, add 350g of hydrazine hydrate, start stirring, heat to 40°C, and keep it for 30h , began to distill off the solvent methanol, then washed with water until neutral, filtered, and drie...
Embodiment 2
[0065] Add 1mol of methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (that is, an ester compound with a hindered phenol structure) and 600ml of methanol into a 1L four-necked flask, pass N 2 , start stirring, and heat in a water bath. When the temperature is constant at 50° C., start to add 200 ml of 30% NaOH solution dropwise. After the dropwise addition, slowly heat to 60°C to make the reaction proceed smoothly. After reacting for 5 hours, transfer to a beaker, then add 1400mL of 2N dilute hydrochloric acid, a white precipitate precipitates, stir for 2 hours, filter, wash and dry to obtain 290g of intermediate acid.
[0066] Add 1 mol of methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate and 3000ml of methanol into a 5L three-necked flask, add 254g of hydrazine hydrate, start stirring, heat to 50°C, and keep it for 22h , began to distill off the solvent methanol, then washed with water until neutral, filtered, and dried to obtain 291 g of the intermediate monohydra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com