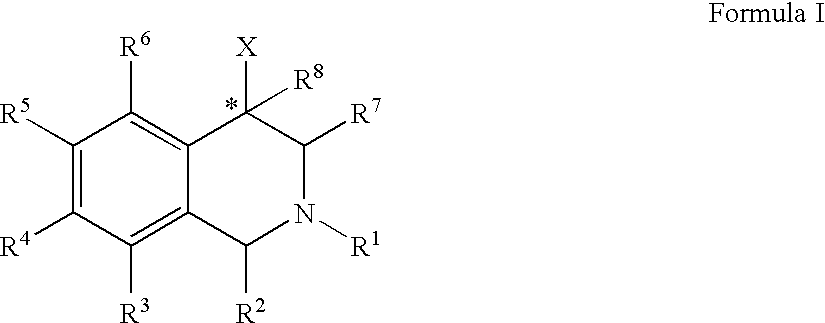
Use of aryl- and heteroaryl-substituted tetrahydroisoquinolines to block reuptake of norepinephrine, dopamine, and serotonin
a technology of aryl- and heteroaryl-substituted tetrahydroisoquinoline and aryl-substituted tetrahydroisoquinoline, which is applied in the direction of drug composition, metabolism disorder, biocide, etc., can solve the problem of discontinuing this drug from the market, affecting the reuptake of norepinephrine, and affecting the reuptake of dopamin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 4-(Benzo[b]thiophen-2-yl)-1,2,3,4-tetrahydroisoquinotine
[1863] Step A: The product from Step A of Example 3 (0.34 g, 1.3 mmol) in CH3OH (10 ml) was stirred for 10 minutes at room temperature under a N2 atmosphere. Sodium cyanoborohydride (0.49 g, 7.8 mmol) was added and stirred for 2 hours. The addition of 3M HCl (4 ml dropwise) turned the reaction from a milky white solution to yellow / green and back to a milky white solution. The mixture was basified with 2N Na2CO3 solution and then concentrated in vacuo to a white solid. The residue was partitioned in H20 (30 ml) and EtOAc (30 ml). The mixture was extracted with EtOAc (3×30 ml). The combined organic layer was washed with brine (50 ml), dried over Na2SO4, filtered and concentrated in vacuo. Chromatography (SiO2, 250 g, 1:49 methanol:EtOAc) afforded the pure product as an oil (0.29 g); HPLC 94.2% purity; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J=7.84 Hz, 1H), 7.61 (d, J=7.76 Hz, 1H), 7.20 (m, 3H), 7.11 (d, J=3.85 Hz, 2H),...
example 2
Preparation of (+)-4-(Benzo[b]furan-2-yl)-2-methyl-1,2,3,4-tetrahydroisoquinoline, hydrochloride salt and (−)-4-(benzo[b]furan-2-yl)-2-methyl-1,2,3,4-tetrahydroisoquinoline, hydrochloride salt
[1865] Racemic 4-(benzo[b]furan-2-yl)-2-methyl-1,2,3,4-tetrahydroisoquinoline was prepared from 2-benzo[b]furanboronic acid and 4-bromoisoquinoline as described in Example 36 (Steps D to F). This racemic compound (120 mg) was separated on semi-prep chiral HPLC (chiralcel OD-H, 1×25 cm, eluent: 3% isopropanol in heptane, flow: 3.1 ml / minute, 500 μl injections, 20 mg / injections). The resulting free bases were dissolved in ethyl acetate and treated with a 2 M solution of HCl in diethyl ether (2 equiv) to give (+)-4-(benzo[b]furan-2-yl)-2-methyl-1,2,3,4-tetrahydroisoquinoline, hydrochloride salt (13 mg, 99.6% AUC HPLC, 100% AUC chiral HPLC): 1H NMR (300 MHz, Acetone-d6) 7.71-7.80 (m, 1H), 7.50-7.58 (m, 1H), 7.31-7.45 (m, 2H), 7.01-7.23 (m, 5H); 5.35-5.50 (m, 1H), 4.47-4.70 (m, 2H), 3.70-4.00 (m, 2...
example 3
Preparation of 4-(Benzo[b]thiophen-2-yl)-2-ethyl-1,2,3,4-tetrahydroisoquinoline, fumarate salt
[1866] Step A: A mixture of 4-bromoisoquinoline (0.64 g, 3.0 mmol) in ethylene glycol dimethyl ether (4 ml), benzothiophene-2-boronic acid (0.69 g, 4.0 mmol), and 2 N Na2CO3 (3 ml) were degassed for 5 minutes then purged under N2 atmosphere for 5 minutes twice. A catalytic amount of Pd(PPh3)4 (0.36 g, 0.3 mmol) was added and the reaction was degassed and purged with N2. The reaction heated to 80° C. with stirring for 12 hours during which the solution turned a dark brown color. The reaction was cooled to room temperature, diluted with H2O (20 ml) and extracted with EtOAc (3×50 ml). The combined organic layer was washed with brine (30 ml), dried with Na2SO4, filtered and concentrated in vacuo. Chromatography (SiO2, 200 g, 1:3 ethyl acetate / hexanes) afforded the pure product as an oil (0.65 g); HPLC 99.5% purity; 1HNMR (500 MHz, CDCl3) δ 9.27 (s, 1H), 8.70 (s, 1H), 8.29 (d, J=8.48 Hz, 1H), 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| anxiety disorder | aaaaa | aaaaa |
| generalized anxiety disorder | aaaaa | aaaaa |
| manic-depressive disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com