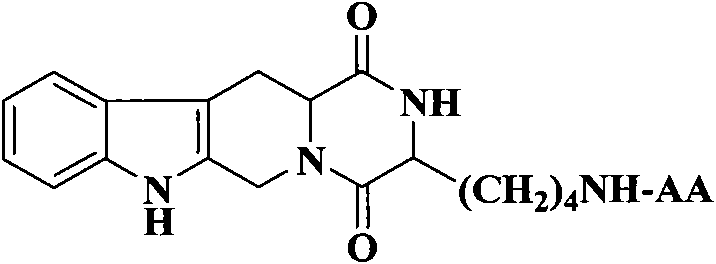
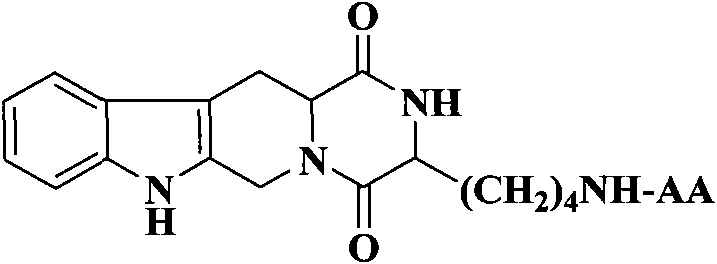
(3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives, and synthesis method and application thereof
A technology of tetrahydrocarboline and 4-b, which can be applied in drug combinations, blood diseases, extracellular fluid diseases, etc., and can solve the problems of poor water solubility of antithrombotic active polycyclic nuclei
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
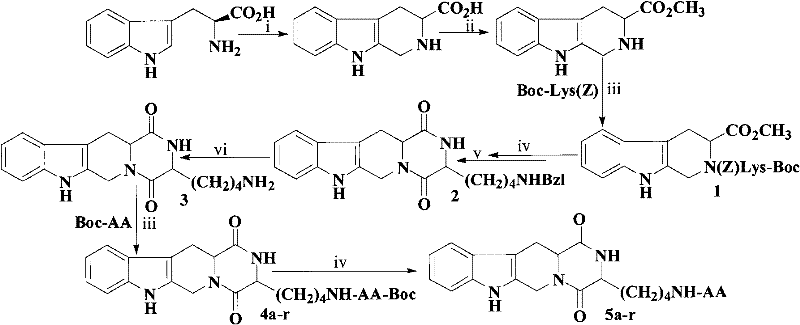
[0038] Example 1 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0039] Put 400ml of water in a 500ml round bottom eggplant bottle, and slowly add 0.2ml of concentrated sulfuric acid. Add 5.0 g (24.5 mmol) of L-tryptophan to the obtained dilute sulfuric acid aqueous solution and ultrasonically shake until the L-tryptophan is completely dissolved. 10 ml of 35% formaldehyde solution was added to the solution, and the reaction mixture was stirred at room temperature for 6 hours. The disappearance of L-tryptophan was detected by thin layer chromatography, and the reaction was terminated. Concentrated ammonia water was slowly added dropwise to the reaction solution, the reaction mixture was adjusted to pH 6, and left to stand for half an hour. The precipitate obtained by suction filtration under reduced pressure was washed with water, and the filtered colorless solid was spread on a petri dish and air-dried to obtain S-carbolinecarboxylic acid, which was 5.0...
Embodiment 2
[0040] Example 2 Preparation of (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester
[0041] 50ml of methanol was cooled to below 0°C with an ice-salt bath, and 10ml (145mmol) of SOCl was added dropwise while stirring 2 10 minutes after the dropwise addition, 5.0 g (23.1 mmol) (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid was added, the ice bath was removed, and the mixture was stirred overnight at room temperature. Stop the reaction after TLC checks that the raw materials basically disappear, and evaporate the solvent methanol and excess SOCl under reduced pressure with a water pump 2 , and then entrained with methanol three times (10ml×3). Add 20ml water and 20ml ethyl acetate to the reaction flask, and add NaHCO 3 Adjust the pH to 7-8, extract the aqueous layer with ethyl acetate (20ml×3), combine the organic layers, wash with saturated sodium chloride three times, and anhydrous NaCl 2 SO 4 After drying and filtering, the filtrate was concentrated...
Embodiment 3
[0042] Example 3 Preparation of (3S)-N-[Boc-(Z)lysyl]-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (1)
[0043] Dissolve 798mg (1.05mmol) Boc-(Z) lysine in 4ml of anhydrous THF, add 433mg (2.10mmol) DCC and 270mg (2mmol) HOBt under ice-cooling, stir for 5-10 minutes and the reaction solution becomes turbid, add 460mg ( 3S)-methyl 1,2,3,4-tetrahydro-β-carboline-3-carboxylate, adjust the pH of the reaction solution to 8-9 with NMM, and remove the ice bath after 30 minutes. After reacting at room temperature for 12 hours, TLC showed that the starting point disappeared, the reaction was stopped, DCU was filtered off, the filtrate was concentrated to dryness at 30°C under reduced pressure, and the residue was dissolved in ethyl acetate. Wash successively with saturated aqueous sodium bicarbonate solution, saturated aqueous sodium chloride solution, 5% aqueous potassium hydrogensulfate solution, saturated aqueous sodium bicarbonate solution, and saturated aqueous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com