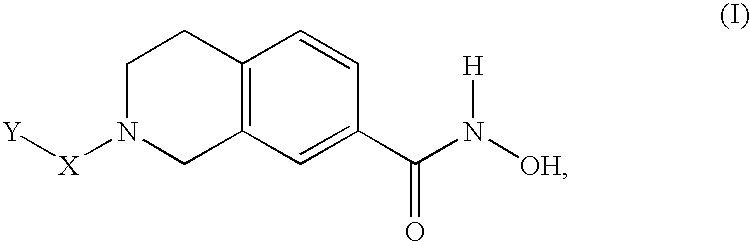
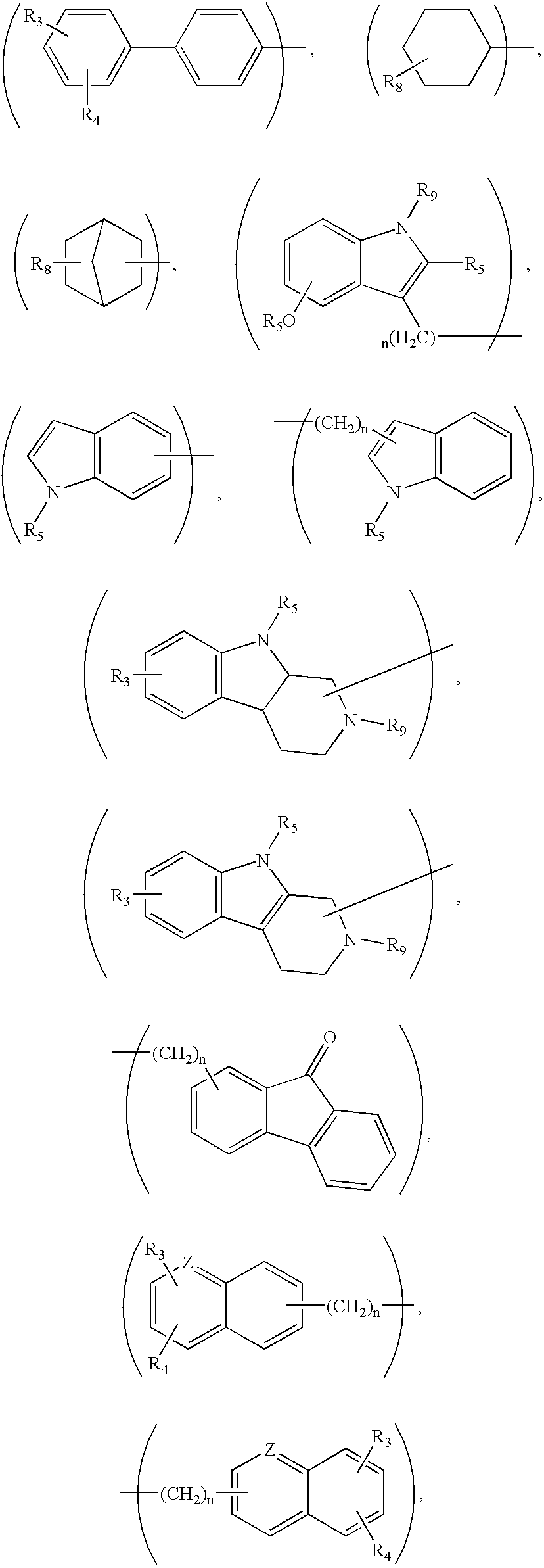
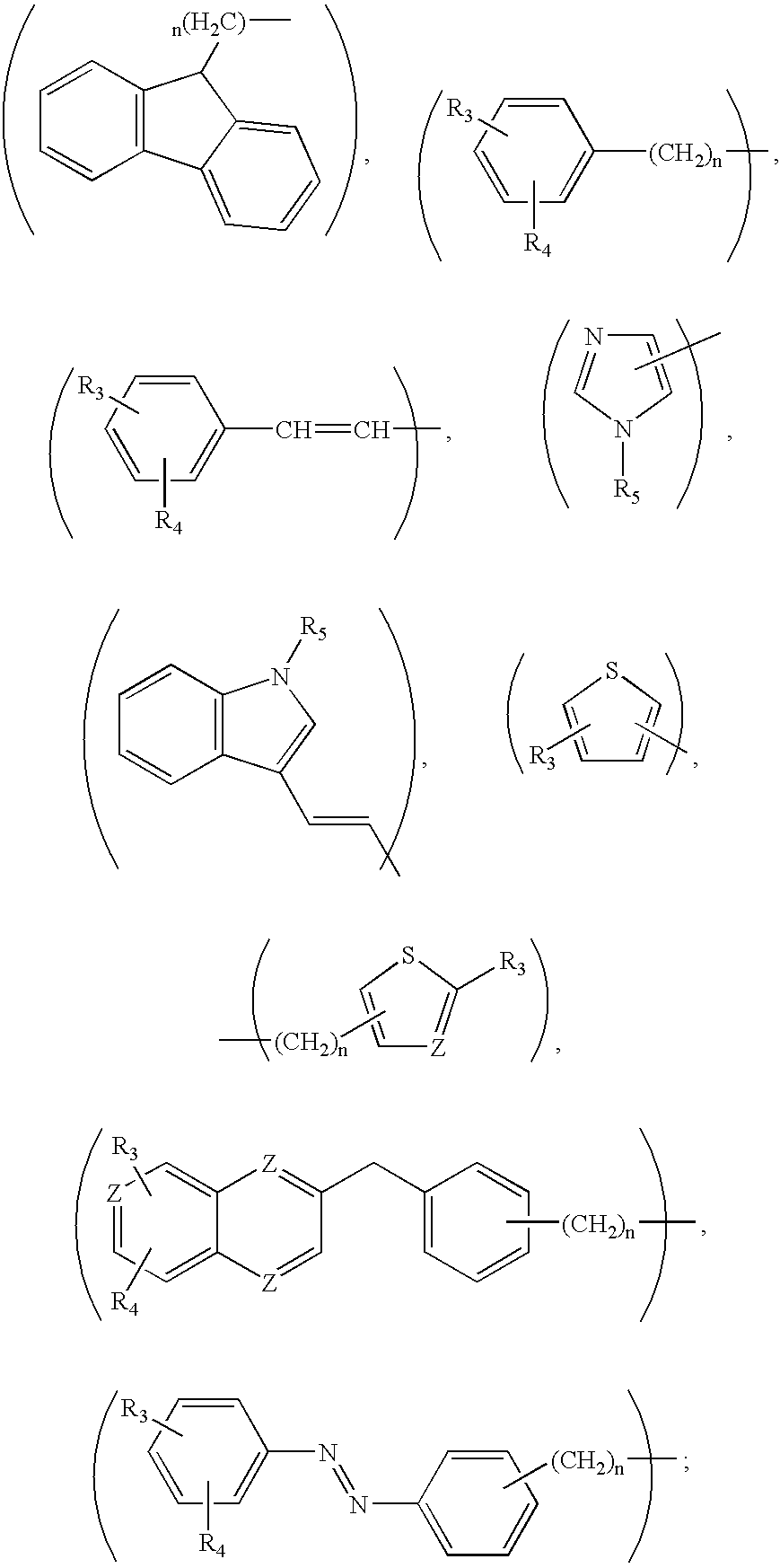
Compounds for treatment of neurodegenerative diseases
a neurodegenerative disease and compound technology, applied in the field of compound treatments for neurodegenerative diseases, can solve the problems that current hdac inhibitors in the art such as tsa and saha may pose limitations in their utility, and achieve the effect of inhibiting deacetylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
7-Nitro-1,2,3,4-tetrahydroisoquinoline hydrochloride (2)
[0105] 1,2,3,4-tetrahydroiosquinoline (Aldrich T1,300-5, 100 gm) (11.6 g, 84.8 mmol) is added dropwise with care to stirred ice-cold concentrated H2SO4 (42.0 mL). Potassium nitrate (9.40 g., 93 mmol) is then added in small portions, taking care that the temperature of the reaction mixture does not rise above 5° C. After stirring overnight at room temperature the dark brown reaction mixture is added carefully to a stirred ice-cold concentrated NH4OH solution. The basic red reaction mixture is extracted with chloroform (three times), and the combined chloroform extracts is washed with brine and dried over anhydrous Na2SO4. Evaporation of the solvent gives a dark brown oil (14.6 g) which was taken up in EtOH (65 mL) and cooled in an ice bath. Treatment of this reddish solution with concentrated HCl (11 mL) yields a viscous yellow precipitate of the hydrochloride salt which is filtered and crystallized from methanol (250 mL) to yi...
example 2
7-Amino-1,2,3,4-tetrahydroisoquinoline dihydrochloride (3)
[0106] 7-Nitro-1,2,3,4-tetrahydroisoquinoline hydrochloride (2) from Example 1, is placed in a Parr shaker bottle (15.0 g, 69.9 mmol) dissolved in 95% EtOH (100 mL), and to the Parr shaker bottle is added concentrated HCl (10 mL), water (25 mL), and PtO2 (0.5 g). The mixture is hydrogenated at 50 psi until no further drop in pressure was observed (about 4 hours). The yellowish suspension is filtered through Celite and evaporated to dryness to afford a yellowish solid which is made basic with 10% NaOH solution (adequate care is exercised in catalyst disposal). Extraction of the basic solution with CHCl3 (three times), followed by drying over anhydrous Na2SO4 and evaporation of the solvent, yields a reddish yellow solid (9.54 g, 92.2%): mp=110-112. 7-Amino-1,2,3,4-tetrahydroisoquinoline dihydrochloride (3) is recrystallized from aqueous MeOH as buff colored needles, mp=290° C.
example 3
7-Cyano-1,2,3,4-tetrahydroisoquinoline Hydrochloride (4)
[0107] 7-Amino-1,2,3,4-tetrahydroisoquinoline dihydrochloride (3) from Example 2 (0.75 g, 5.1 mmol) is dissolved in concentrated HCl (1.75 mL) and water (2 mL) and stirred in an ice bath, giving a red solution. To this solution is added dropwise NaNO2 (0.35 g, 5.1 mmol) dissolved in water (2 mL). After 15 minutes stirring, a positive starch-iodide test is obtained and the excess HNO2 is destroyed by the addition of urea (0.10 g).
[0108] In a second flask, a solution of NaOH (0.50 g in 1.5 mL water) and KCN (1.63 g in 5 mL of water) is prepared, and benzene (5 mL) is added. The suspension is chilled in an ice bath, and to it is added a solution of Ni2SO4.6H2O (1.3 g, 5 mmol) in 2.5 mL water). The color of the resulting mixture changes to yellow-brown. To this mixture is added dropwise with vigorous stirring the diazotized solution. Brisk evolution of N2 is observed, and the reaction mixture is allowed to warm to room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polymeric | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com