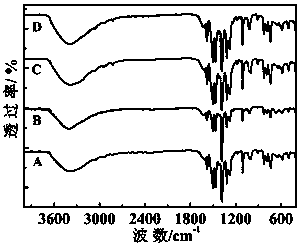
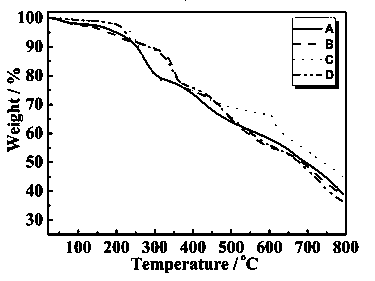
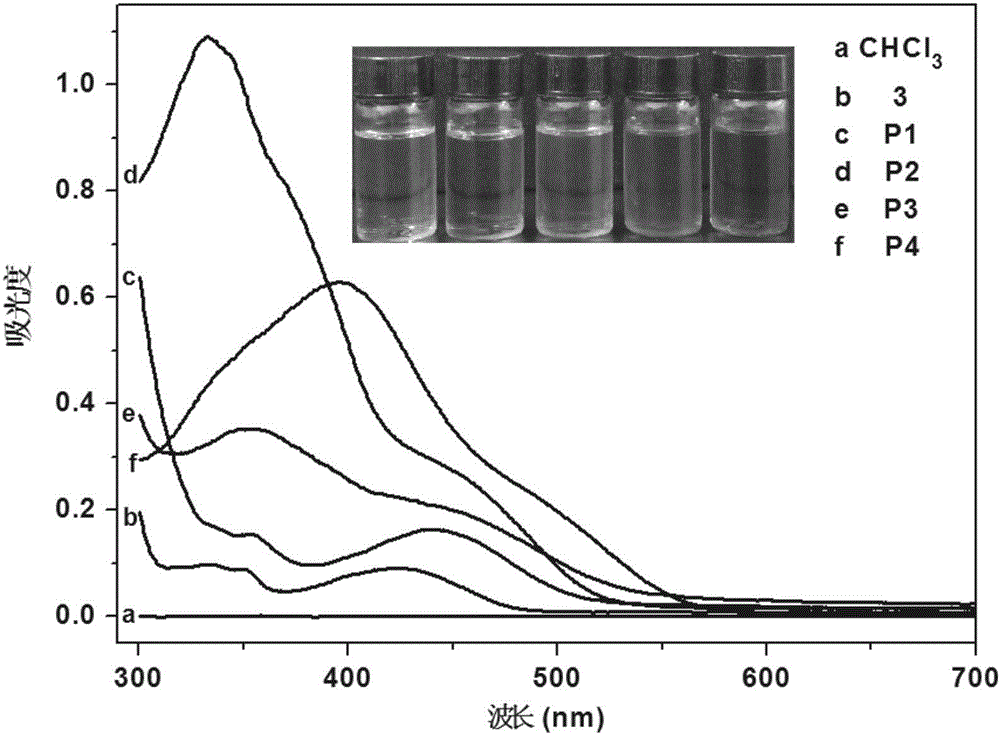
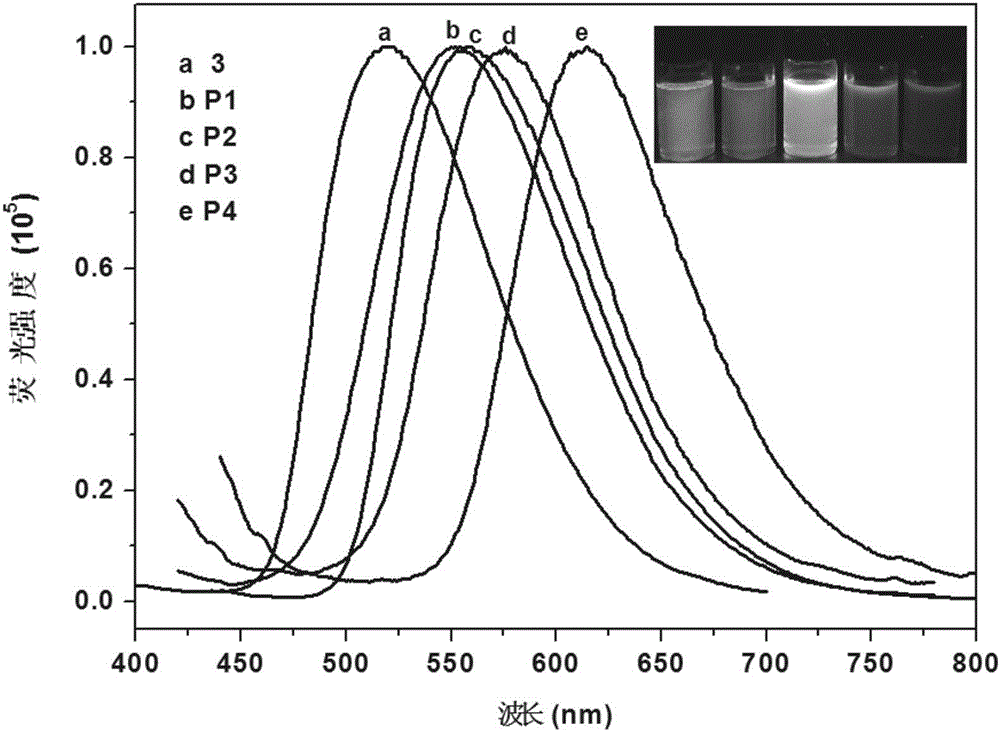
Patents
Literature
537 results about "Hydroxyquinolines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
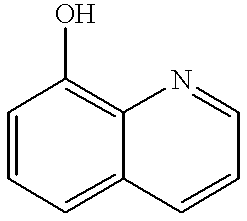
The 8-hydroxy derivatives inhibit various enzymes and their halogenated derivatives, though neurotoxic, are used as topical anti-infective agents, among other uses.
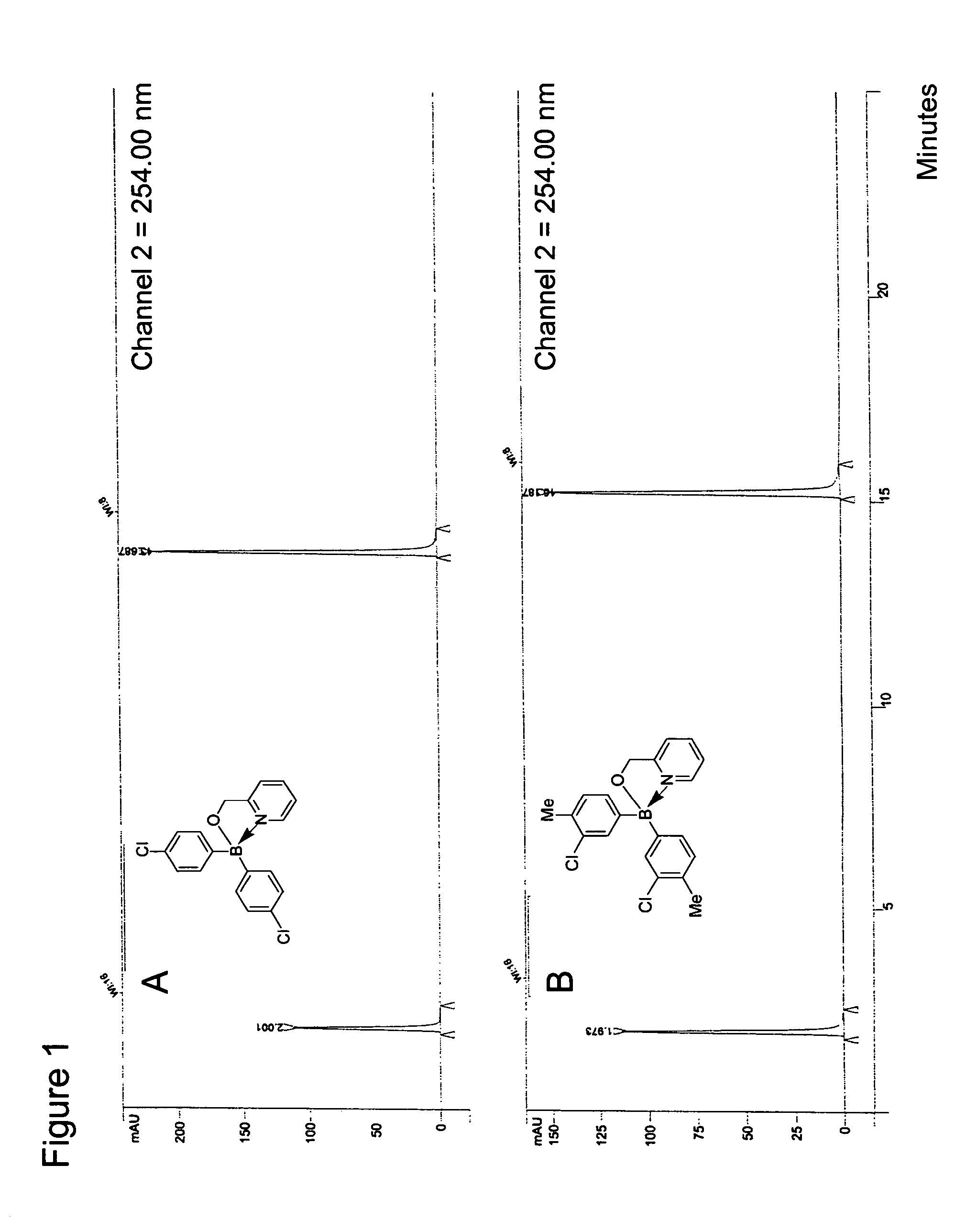
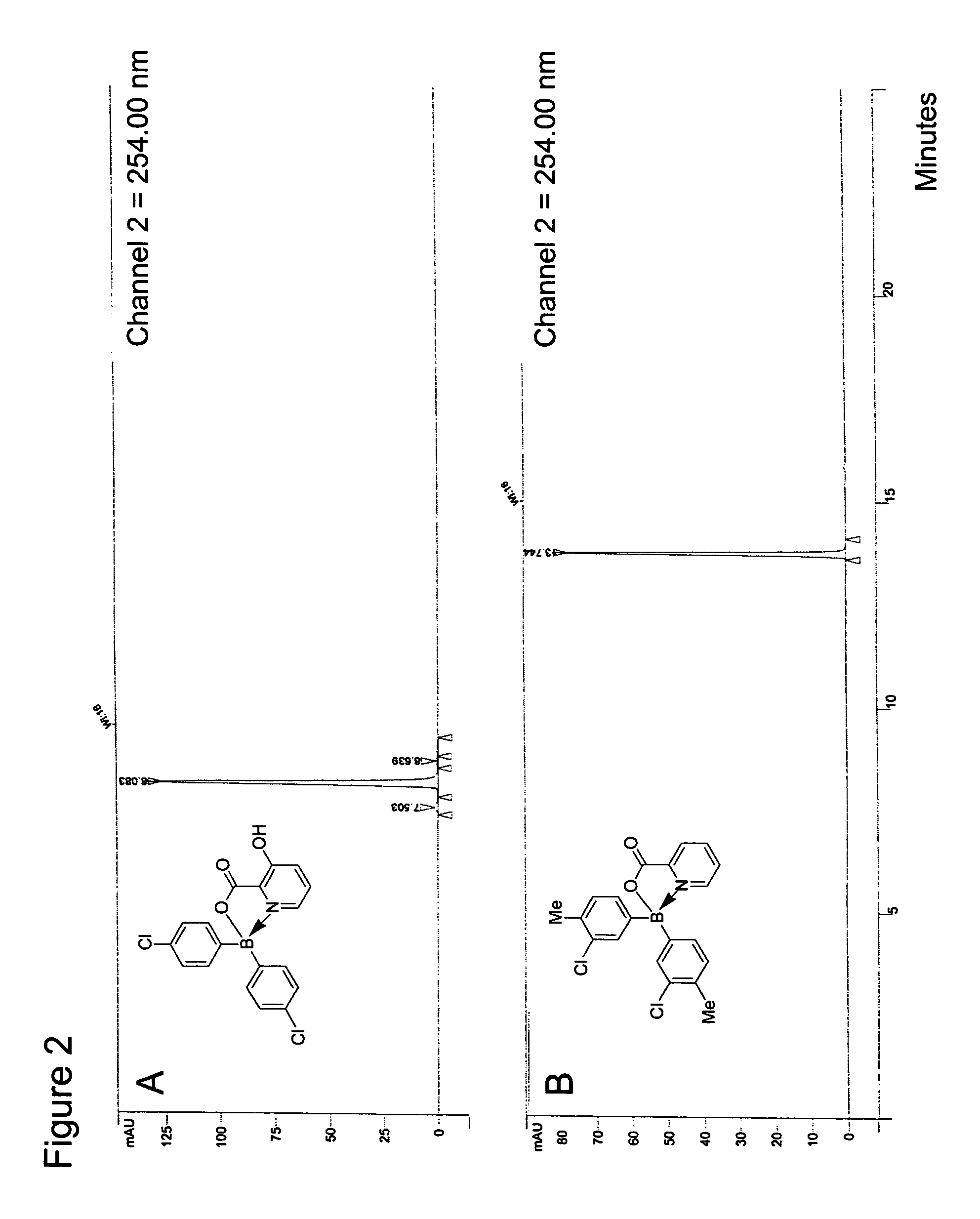
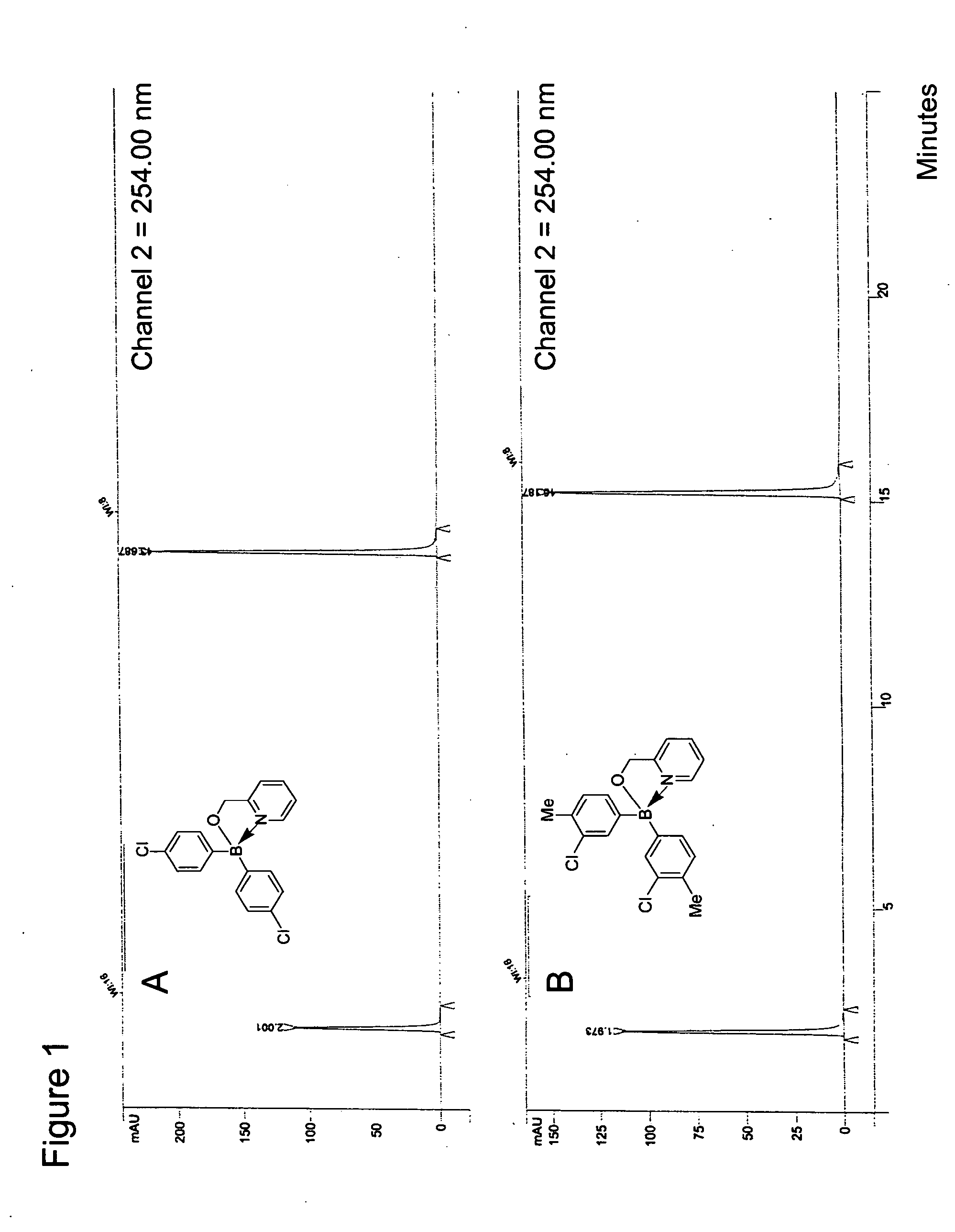
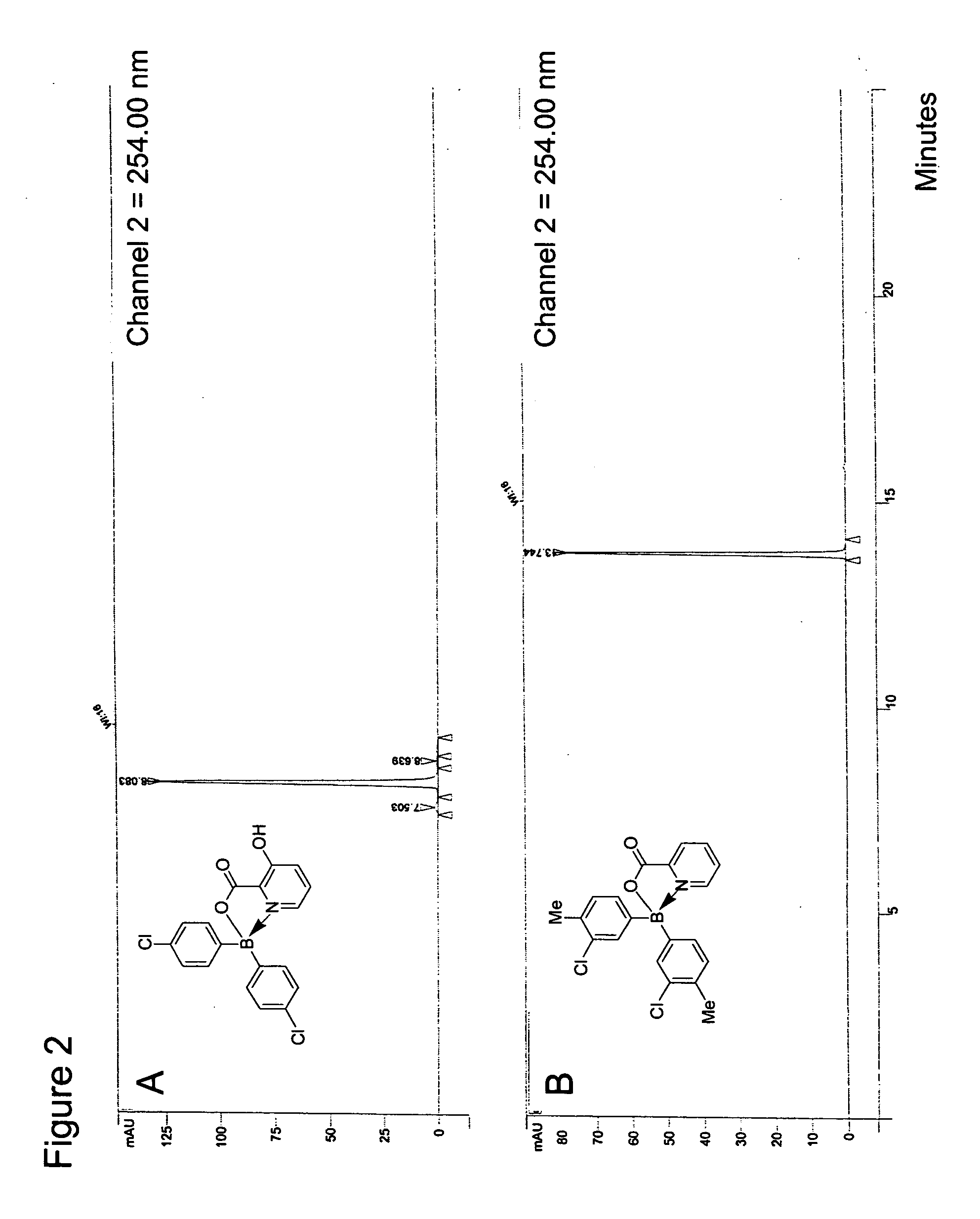
Antibiotics containing borinic acid complexes and methods of use
The structure and preparation of antibiotics incorporating borinic acid complexes are disclosed, especially hydroxyquinoline, imidazole and picolinic acid derivatives, along with compositions of these antibiotics and methods of using the antibiotics and compositions as bactericidal and fungicidal agents as well as therapeutic agents for the treatment of diseases caused by bacteria and fungi.
Owner:ANACOR PHARMA INC
Polishing composition and method for defect improvement by reduced particle stiction on copper surface
InactiveUS20060278614A1Decorative surface effectsSemiconductor/solid-state device manufacturingPhysical chemistryEther
Owner:CABOT MICROELECTRONICS CORP
Bis(hydroxyquinoline) metal complexes as bleach catalysts
InactiveUS20100048447A1Non-ionic surface-active compoundsOrganic detergent compounding agentsPtru catalystPhysical chemistry
Owner:HENKEL KGAA
Compounds for treatment of neurodegenerative diseases
InactiveUS20060004041A1Potential limitationInhibit deacetylationBiocideOrganic chemistryHydroxamic acidNeuro-degenerative disease
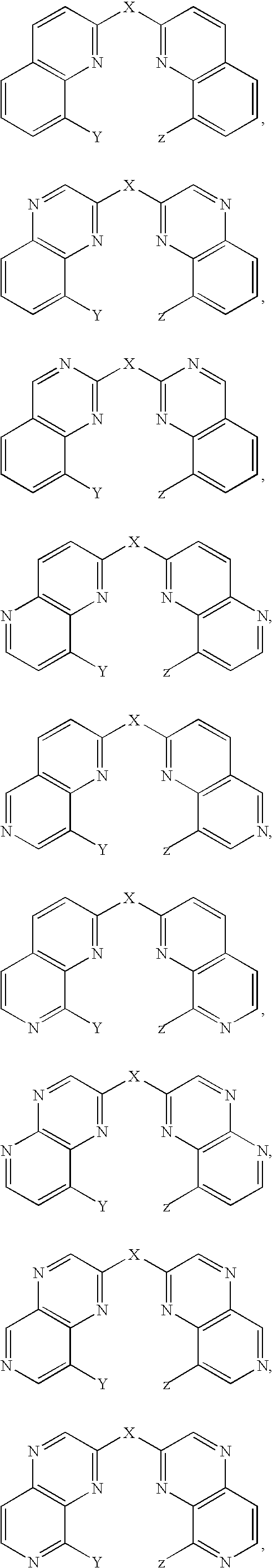
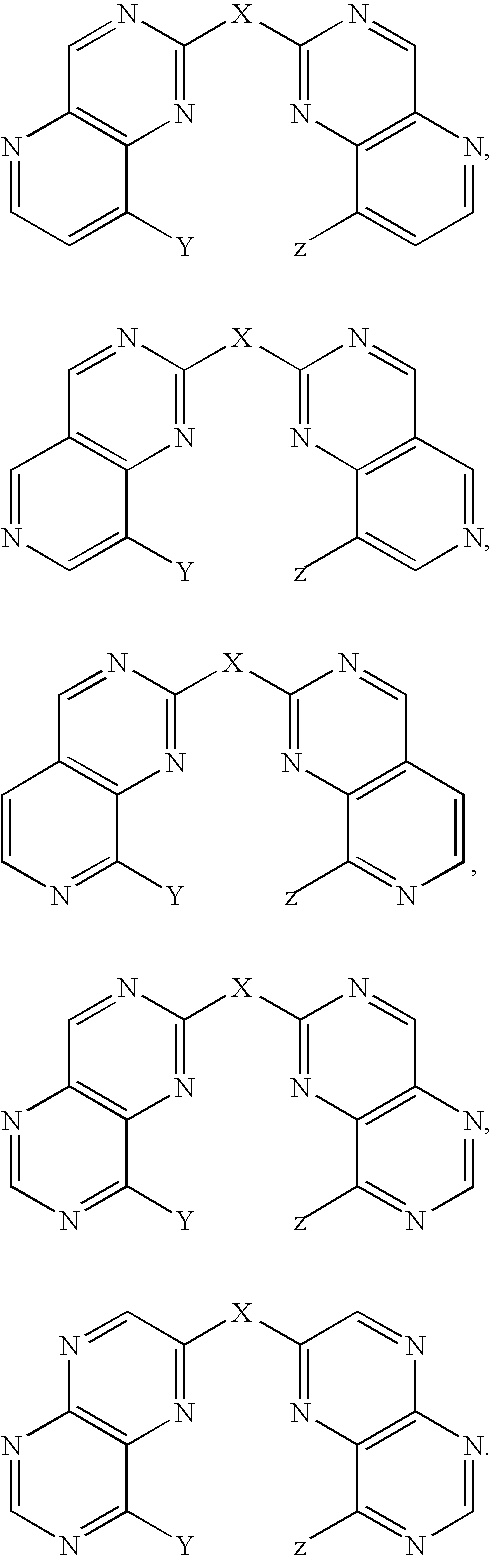
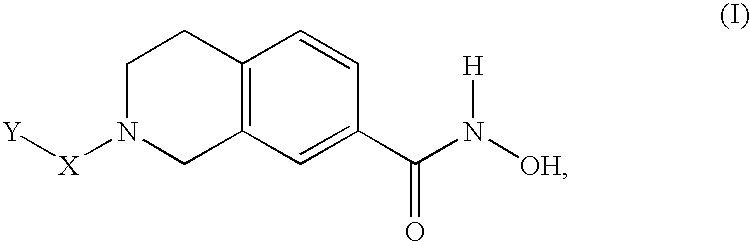
The present invention relates to a class of small molecule hydroxamic acid compounds capable of inhibiting histone deacetylases (HDACs). The present invention also relates to methods of preparation of hydroxamic acid HDAC inhibitor compounds of the invention, which are N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, and their incorporation into pharmaceutical compositions and methods of administration. The present invention also relates to N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, which may be prepared as a hydroxamic acid HDAC inhibitor compound library that can be utilized in screening methods known in the art.
Owner:FORUM PHARMA
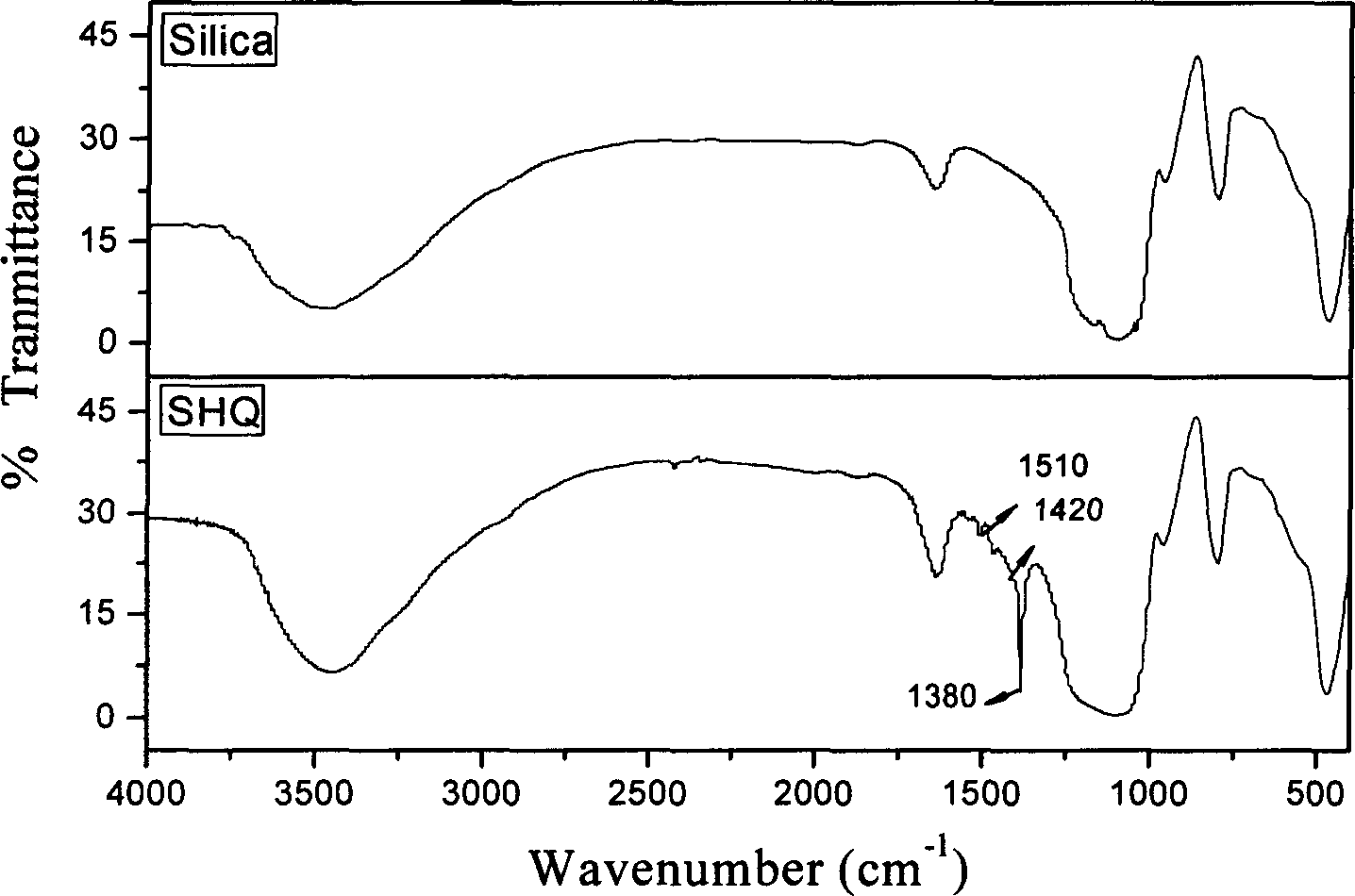
8-hydroxy quinoline type chelated resin and its synthesis
InactiveCN1772386AImprove rigidityImprove the coordination effectComplex ion-exchangersIndustrial waste waterQuinoline
The 8-hydroxy quinoline type chelated resin has 8-hydroxy quinoline fixed onto macroporous silica gel through silanizing reagent and paraformaldehyde as spacer arm and bonding amount of 120-542 umol / g. Its synthesis process includes the steps of: pre-treating macroporous silica gel; introducing spacer arm; fixing ligand and acid washing to set via drying intermediate product II, washing with alcohol and drying for several times, washing with hydrochloric acid, and washing with deionized water to neutral so as to obtain yellow chelated resin. The resin has simple synthesis process, mild reaction condition, short synthesis time, easy control, high synthesis efficiency, low cost, high rigidity and high metal ion complexing capacity, and may be used in on-line enriching analysis of trace metal, enriching recovery of noble metals, purifying industrial waste water, etc.
Owner:THE FIRST INST OF OCEANOGRAPHY SOA
Computer-to-plate by ink jet
InactiveUS20020043171A1Simple and inexpensive methodProduce littleDuplicating/marking methodsHand compositionComputer to plateHydroxybenzotriazole
A method for preparing a lithographic printing plate by means of ink jet is disclosed. The ink jet fluid contains an oleophilizing compound as defined in the description. Preferred compounds are 8-hydroxyquinolines, 7-hydroxybenzimidazoles, and 7-hydroxybenztriazoles.
Owner:AGFA NV
Neuroprotective iron chelators and pharmaceutical compositions comprising them
Novel iron chelators exhibiting neuroprotective and good transport properties are useful in iron chelation therapy for treatment of a disease, disorder or condition associated with iron overload and oxidative stress, eg. a neurodegenerative or cerebrovascular disease or disorder, a neoplastic disease, hemochromatosis, thalassemia, a cardiovascular disease, diabetes, a inflammatory disorder, anthracycline cardiotoxicity, a viral infection, a protozoal infection, a yeast infection, retarding ageing, and prevention and / or treatment of skin ageing and skin protection against sunlight and / or UV light. The iron chelator function is provided by a 8-hydroxyquinoline, a hydroxypyridinone or a hydroxamate moiety, the neuroprotective function is imparted to the compound e.g. by a neuroprotective peptide, and a combined antiapoptotic and neuroprotective function by a propargyl group.
Owner:TECHNION RES & DEV FOUND LTD +1
Anticancer composition and its application
InactiveCN102274347AImprove the quality of lifeProlong survival timeInorganic active ingredientsAntineoplastic agentsHuman tumorSide effect
The invention relates to an anticancer composition and application thereof. The anticancer composition comprises dithioamides or / and oxyquinolines with metallic element combining capacity, organic or / and inorganic salts containing copper or / and zinc, Chinese herbal medicine extract and pharmaceutically acceptable carrier or solvent, wherein the Chinese herbal medicine comprises at least one of ginseng, radix astragali, wolfberry fruit, mulberry, Rabdosia rubescens, gromwell, radix salviae miltiorrhizae, pseudo-ginseng, peach kernel, eucommia and oldenlandia. The Chinese herbal medicine in the anticancer composition can obviously enhance the inhibiting action of the dithioamides or / and oxyquinolines + copper or / and + zinc on human tumor cells, improve the treatment effect of inducing apoptosis of tumor cells, reduce the toxic and side effect and obviously inhibit tumor growth, thereby enhancing the life quality of the patients with tumors and prolonging the survival time of the patients with tumors.
Owner:陈迪 +1
Ruthenium complex capable of inhibiting tumor angiogenesis and preparation method and application thereof
InactiveCN102516309AImprove stabilityImprove solubilityOrganic active ingredientsGroup 8/9/10/18 element organic compoundsSolubilityLithium chloride
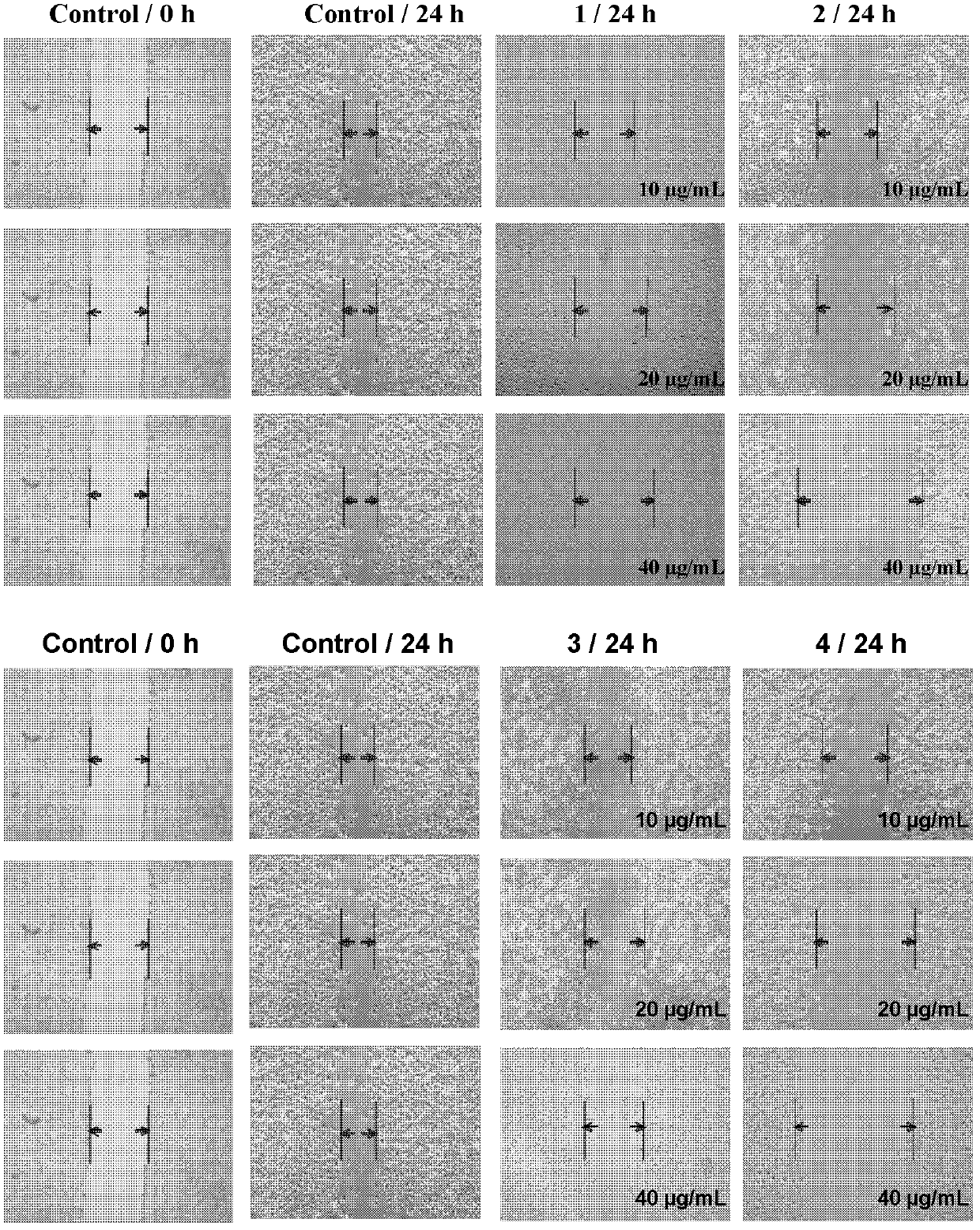
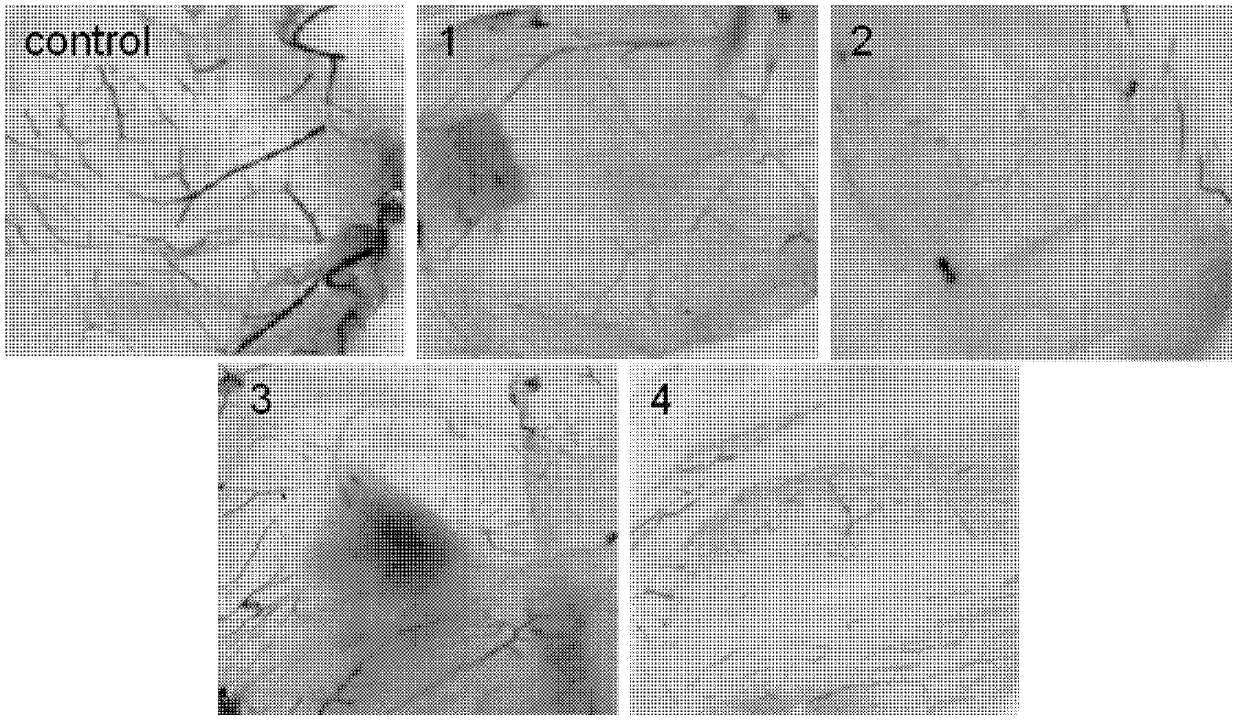
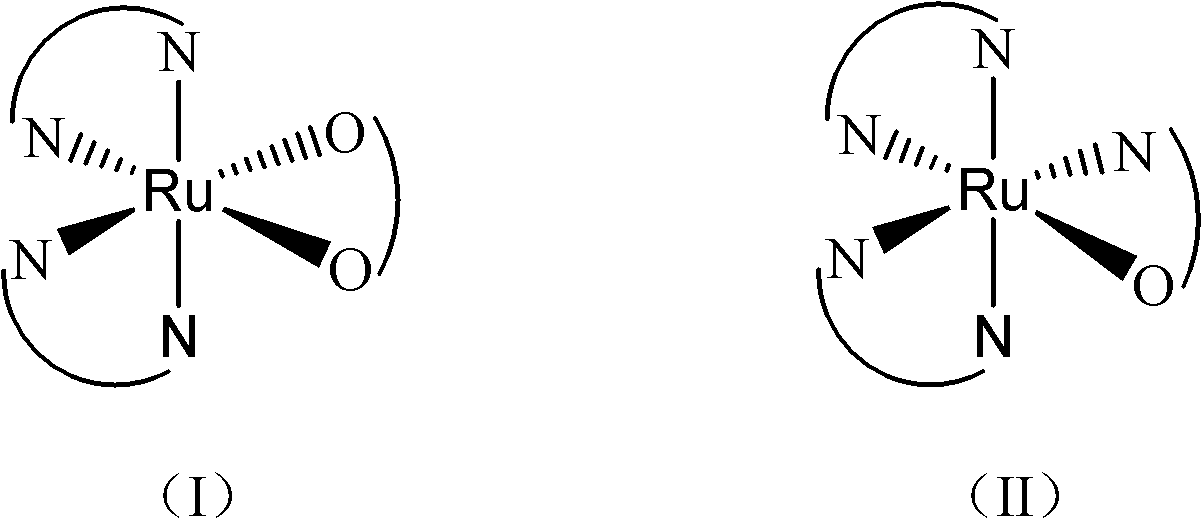
The invention belongs to the field of chemical drugs, and discloses a ruthenium complex capable of inhibiting tumor angiogenesis and a preparation method and application thereof. The ruthenium complex provided by the invention has a structure shown in a formula I or II. The preparation method comprises the following steps of: dropwise adding a silver nitrate solution to a sodium salt solution, stirring and reacting, then filtering, washing and vacuum drying a precipitate, and thereby obtaining a ligand O-O; taking ruthenium chloride, L, and lithium chloride to be dissolved in N-, N-dimethyl formamide, heating and refluxing under the protection of argon atmosphere to obtain an intermeidate Ru (L2) Cl22 +; allowing the ligand O-O and the Ru (L2) Cl22 + to be dissolved in an ethanol / water mixed solvent, heating and refluxing to obtain the ruthenium complex shown in the formula I; and allowing the Ru (L2) Cl22 +, 8- hydroxyquinoline, and ammonium acetate to be dissolved in ethanol, and heating and refluxing under the protection of argon atmosphere to obtain the ruthenium complex shown in the formula II. The ruthenium complex has the advantages of good stability, uneasiness in hydrolysis, good solubility, low toxicity, and the ability to inhibit tumor angiogenesis, and is easily absorbed by the human body.
Owner:JINAN UNIVERSITY
Anti-viral uses of borinic acid complexes
Owner:ANACOR PHARMA INC
Environment-friendly high effective fresh-keeping agent for cutting flower
InactiveCN1843112AFormula rich in ingredientsComprehensiveDead plant preservationGrowth plantInorganic salts
The invention relates to a flower antistaling agent with added plant growth regulator, wherein 1L of the water contains the following constituents: sucrose 5-20g, 8- hydroxyquinoline 0.1-0.5g, gibberellin 0.02-0.08g, cytokinin 0.01-0.09g, plant growth retardant 0.20-0.8g, arginine 0.015-0.045g, organic acid 0.1-0.5g and inorganic salt 0.005-1.5g.
Owner:JIANGHAN UNIVERSITY
Red organic electroluminescent device and preparation thereof
ActiveCN101384112AImprove transmission performanceWiden the luminous rangeElectrical apparatusElectroluminescent light sourcesPhenanthroline8-Hydroxyquinoline
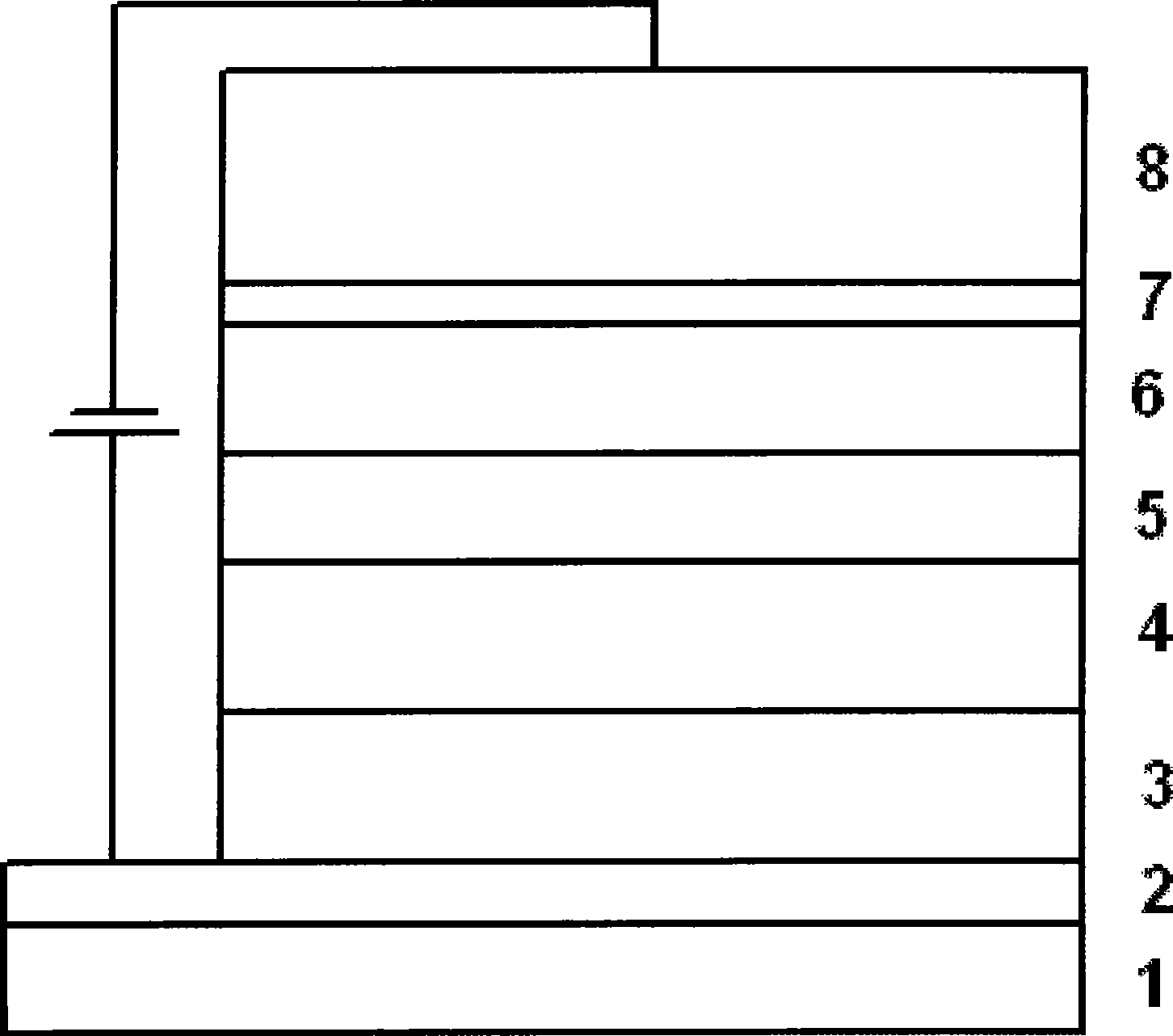
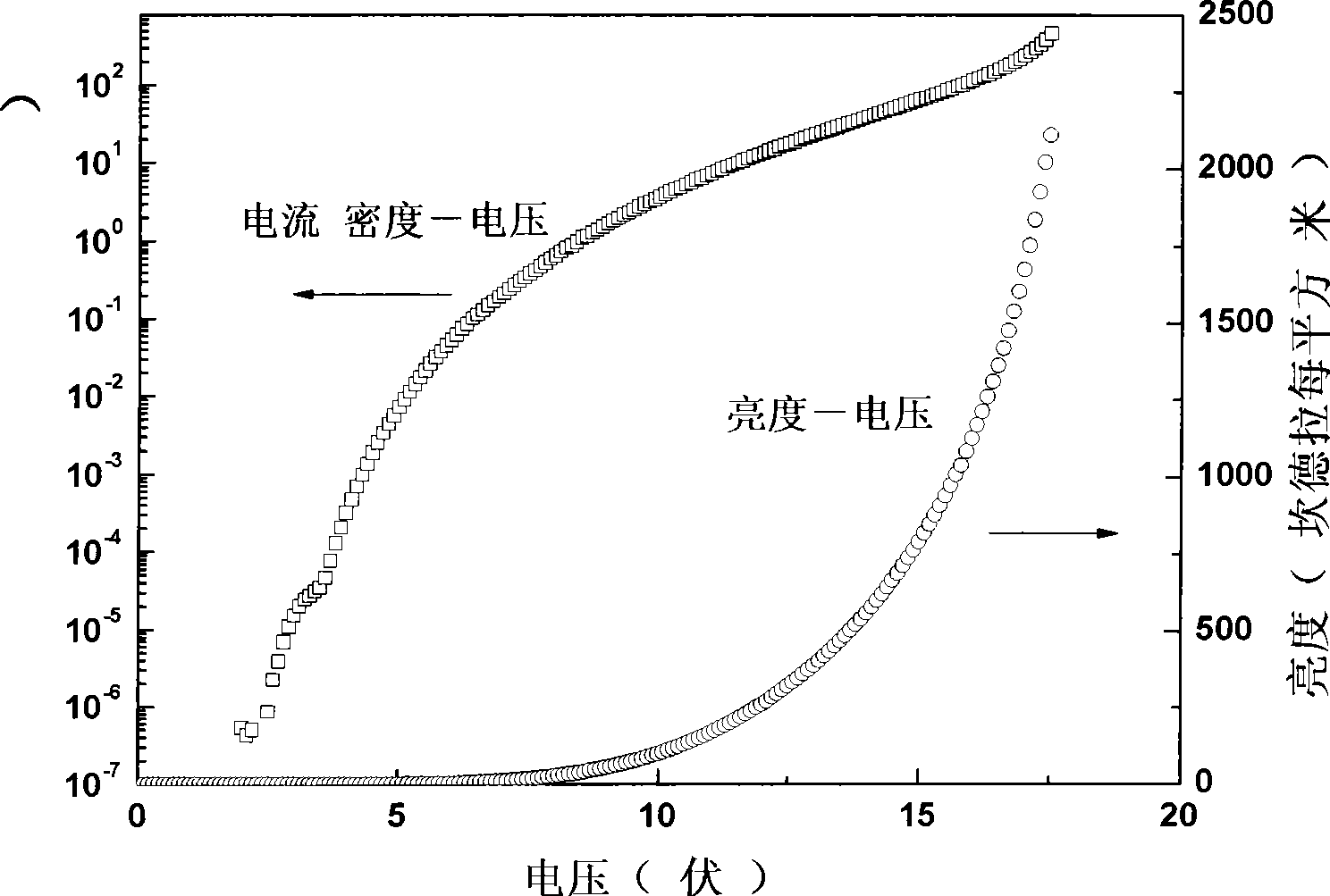
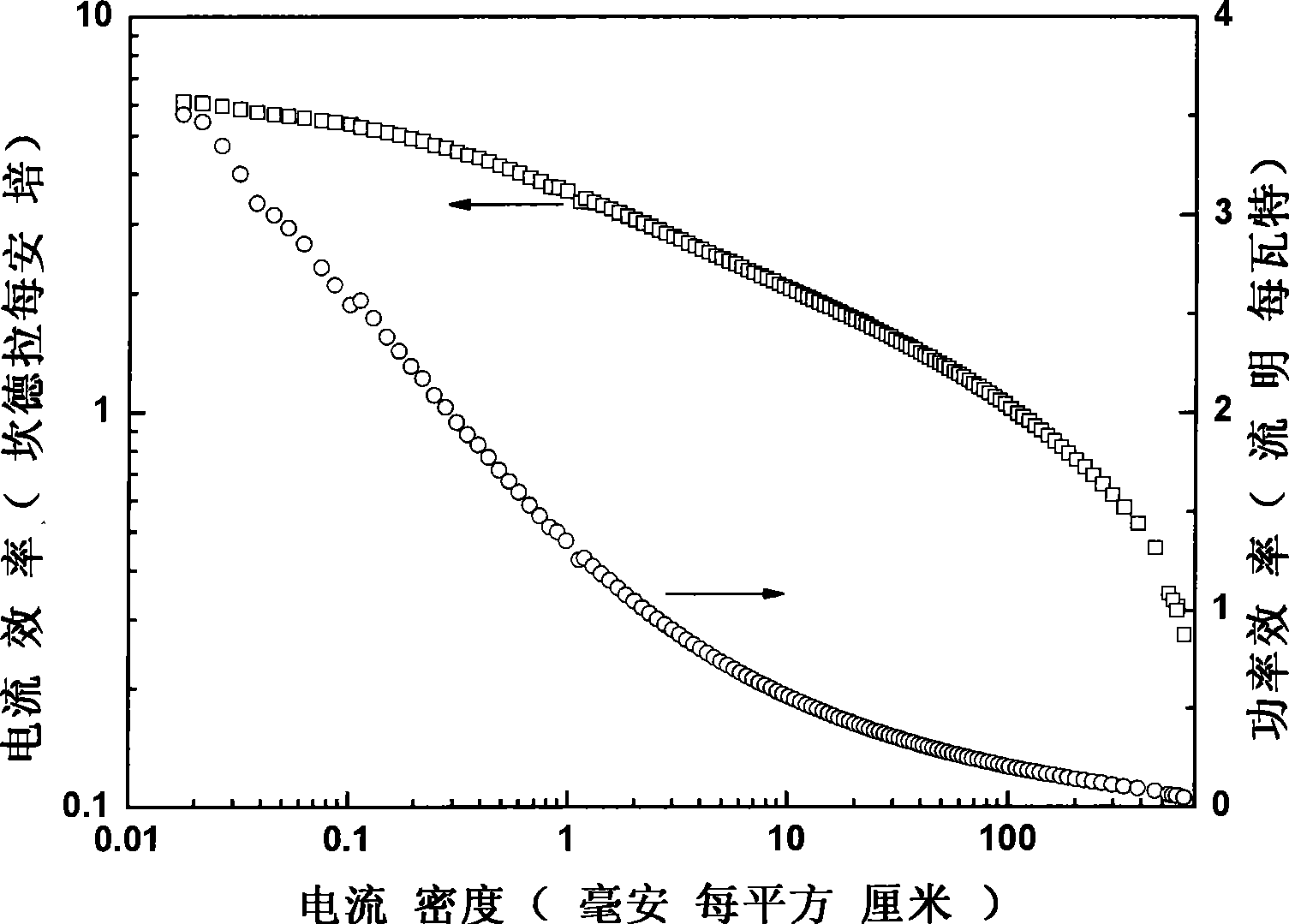
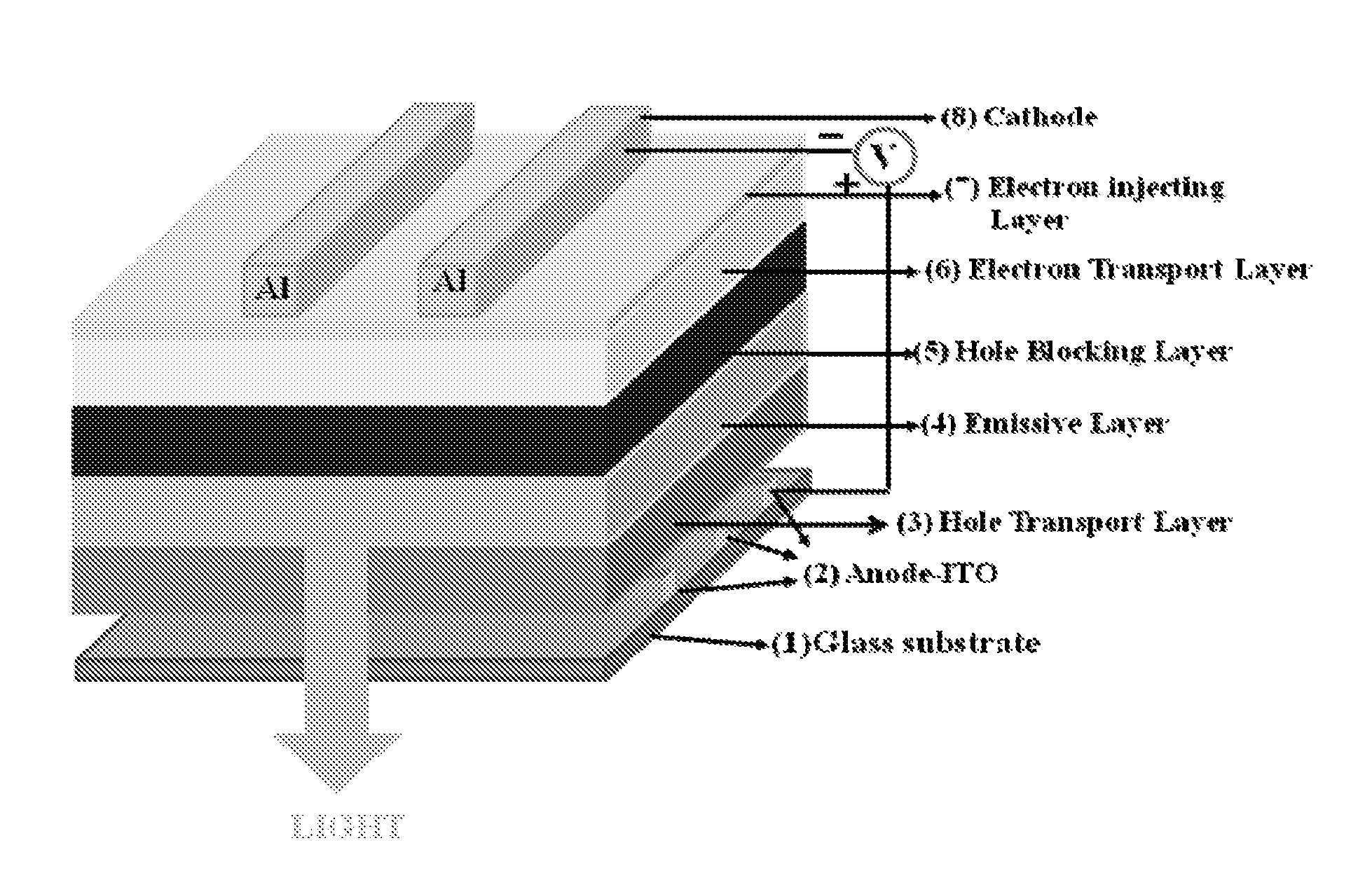
The invention belongs to a red organic electroluminescence device and a preparing method thereof. A vacuum evaporation technology is adopted to prepare indium tin oxide / 4, 4'-twin [N-(p-methylphenyl)-N-phenyl-amino] diphenyl or N, N'-double(1- naphthyl)-N, N'-biphenyl-1, 1'-biphenyl-4 and 4'-diamine / 8-hydroxyquinoline aluminum, classical trivalence europium composition Eu (TTA)3phen taking trifluoroacetylacetone thiofuran and BPT as a first ligand and a second ligand, and a main material 4, 4'-N, N'-twin carbazole biphenyl / 2, 9- dimethyl-4, 7- dimethyl-1, 10- phenanthroline / 8- hydroxyquinoline aluminum / lithium fluoride / aluminum. The ultimate electroluminescence current efficiency of the device is 6.1cd / A, the ultimatepower efficiency is 3.5lm / W, and the ultimate brightness is 2451.69cd / m<2>.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Computer-to-plate by ink jet
InactiveUS6457413B1Simple and inexpensive methodProduce littleDuplicating/marking methodsPrinting pre-treatmentComputer to plateEngineering
A method for preparing a lithographic printing plate by means of ink jet is disclosed. The ink jet fluid contains an oleophilizing compound as defined in the description. Preferred compounds are 8-hydroxyquinolines, 7-hydroxybenzimidazoles, and 7-hydroxybenztriazoles.
Owner:AGFA NV
Antibiotics containing borinic acid complexes and methods of use
The structure and preparation of antibiotics incorporating borinic acid complexes are disclosed, especially hydroxyquinoline, imidazole and picolinic acid derivatives, along with compositions of these antibiotics and methods of using the antibiotics and compositions as bactericidal and fungicidal agents as well as therapeutic agents for the treatment of diseases caused by bacteria and fungi.
Owner:ANACOR PHARMA INC
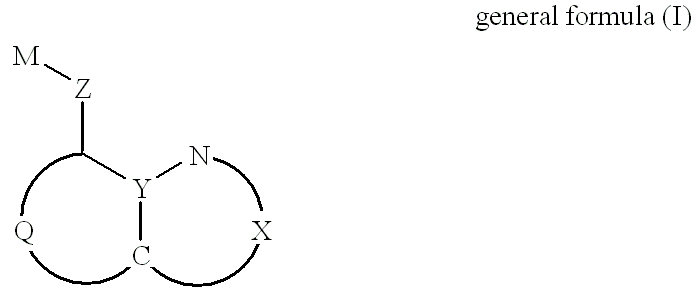
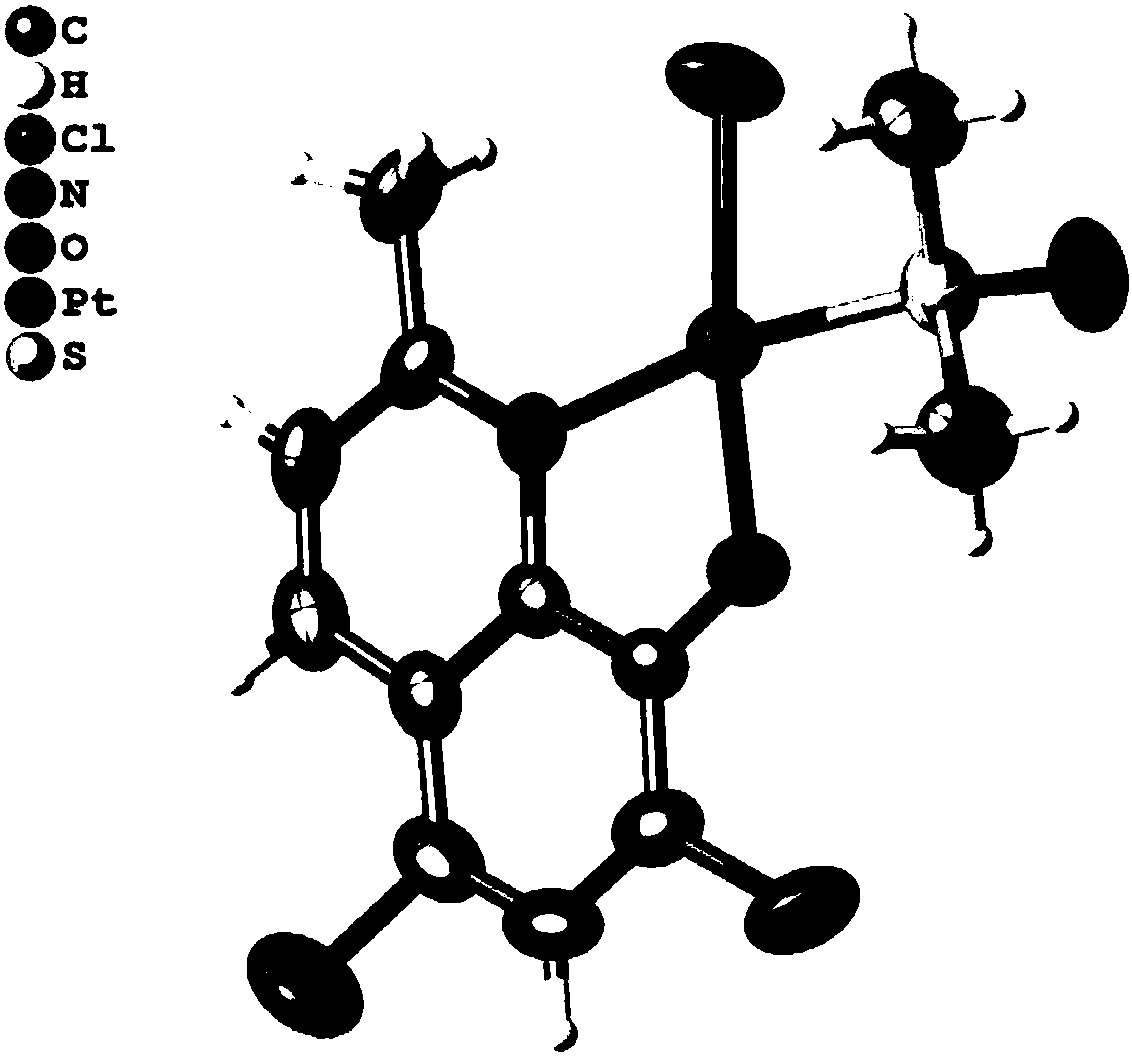
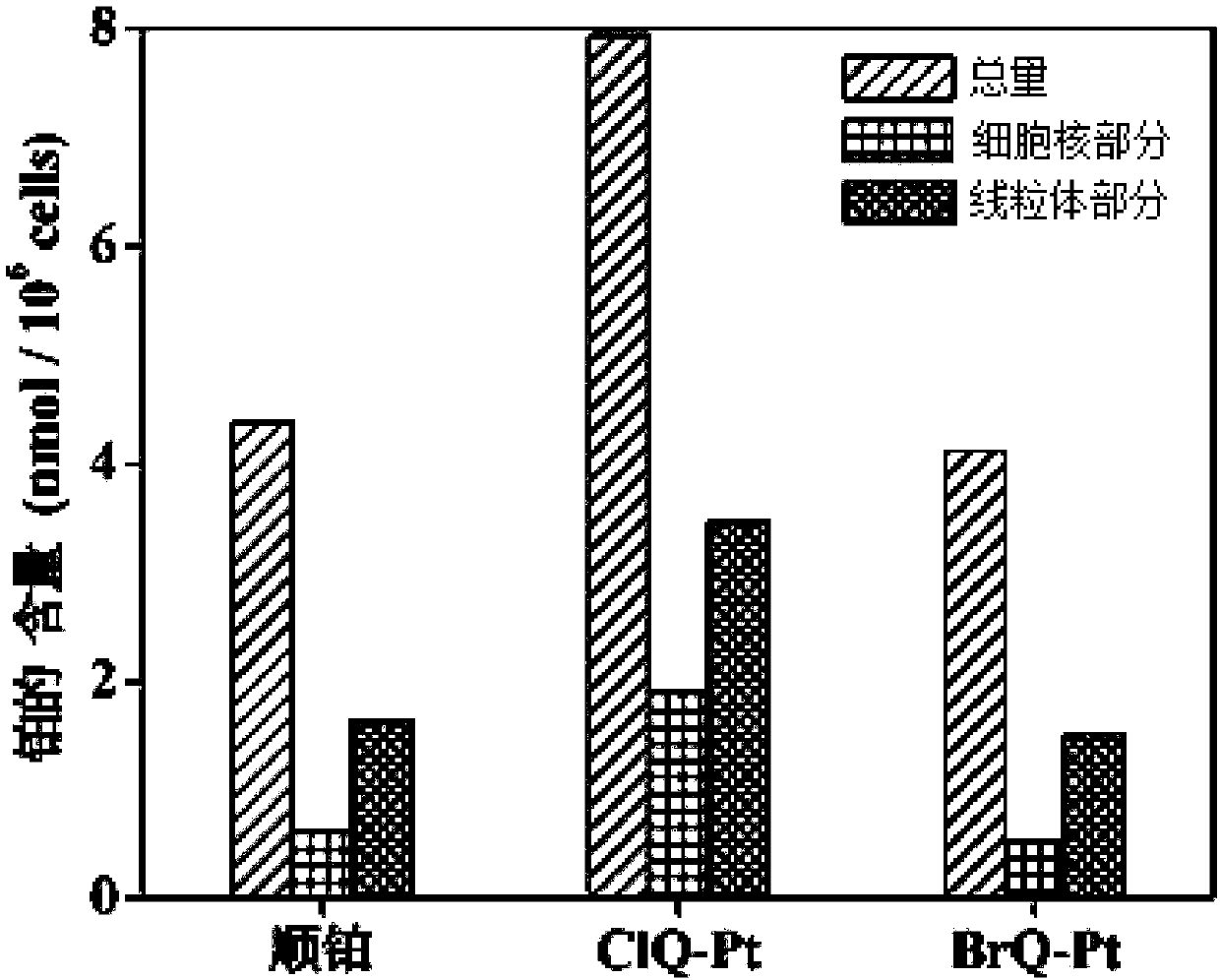
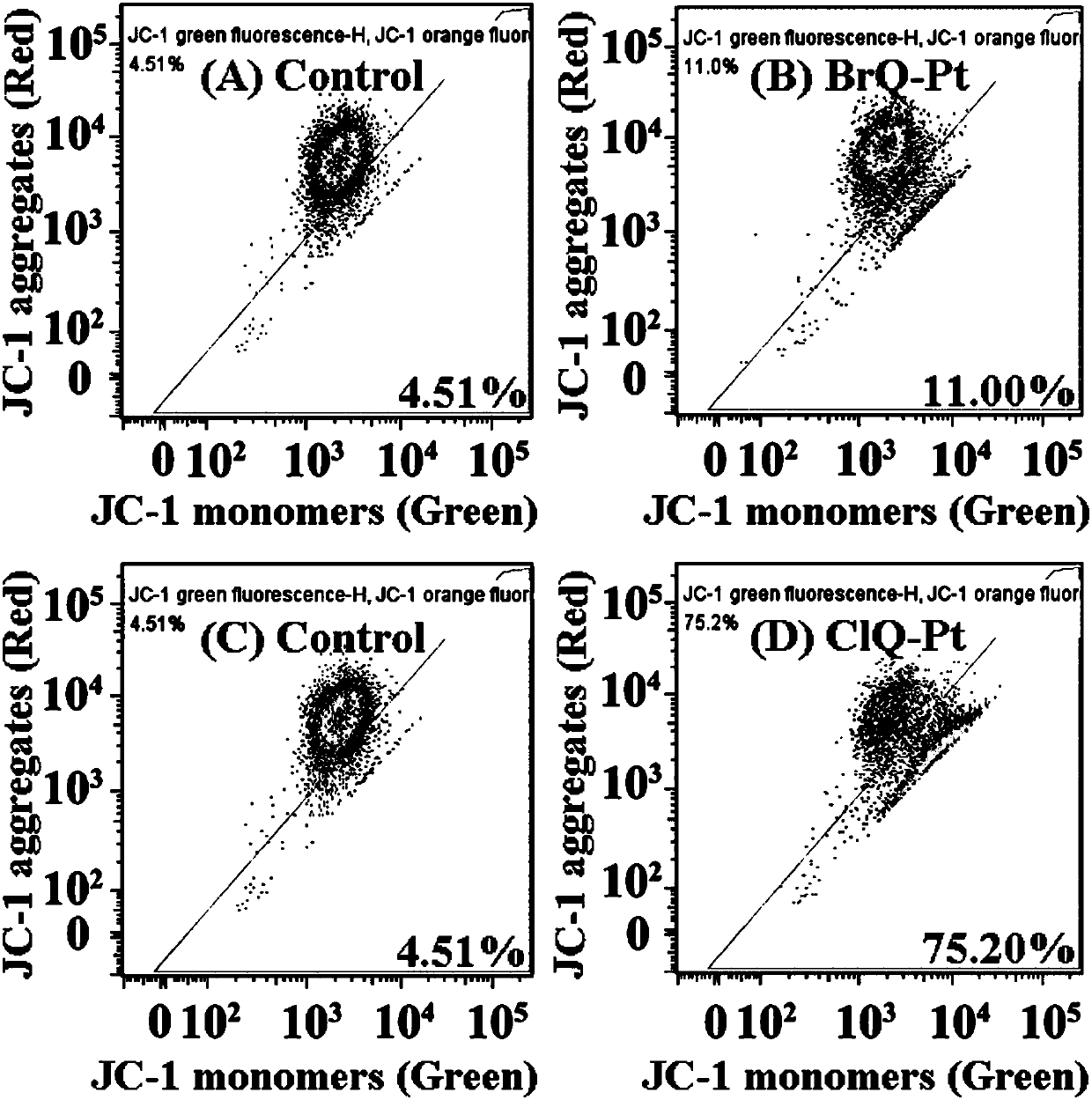
Halogenated 8-hydroxyquinoline platinum (II) complexes and synthesis method and application thereof
InactiveCN108191918AStrong antiproliferative activityGrowth inhibitionOrganic active ingredientsGroup 8/9/10/18 element organic compoundsBladder cancerPlatinum
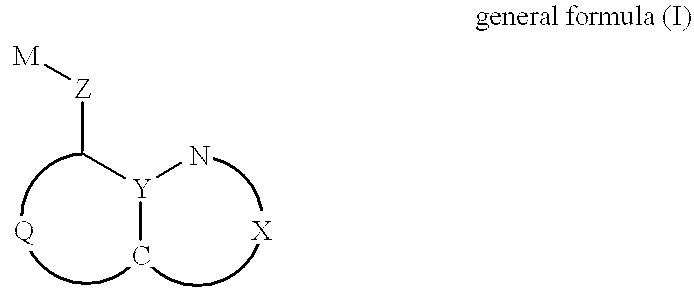
The invention discloses two halogenated 8-hydroxyquinoline platinum (II) complexes and a synthesis method and application thereof. The structures of the halogenated 8-hydroxyquinoline platinum (II) complexes are shown in the formula (I), and the synthesis method comprises the steps that an 8-hydroxyquinoline derivative and dichloro bis(dimethyl sulfoxide) platinum are taken and subjected to a coordination reaction in a polar solvent, and the halogenated 8-hydroxyquinoline platinum (II) complexes are obtained. It is shown through experiments carried out by an applicant that the halogenated 8-hydroxyquinoline platinum (II) complexes have good activity of inhibiting the proliferation of certain tumor cell strains and can selectively inhibit the growth of bladder cancer T-24 cells, and the apoptosis of the tumor cells is induced under the function of targetedly interfering with mitochondria; meanwhile, the toxicity of the complexes to human normal cells HL-7702 is low. The structures of the halogenated 8-hydroxyquinoline platinum (II) complexes and the structure of the 8-hydroxyquinoline derivative are shown in the following formulas (I) and (II) respectively, wherein the formulas (I)and (II) are shown in the description, and R1 represents Cl or Br.
Owner:GUILIN NORMAL COLLEGE
Cut-flower antistalling agent
An antistaling agent for fresh flower is prepared from water as solvent, cane sugar, 8-hydroxyquinoline sulfate or 8-hydroxyquinoline citrate or thiabendazole, silver thiosulfate, cytokinin and citric acid.
Owner:辽宁省农业科学院园艺研究所
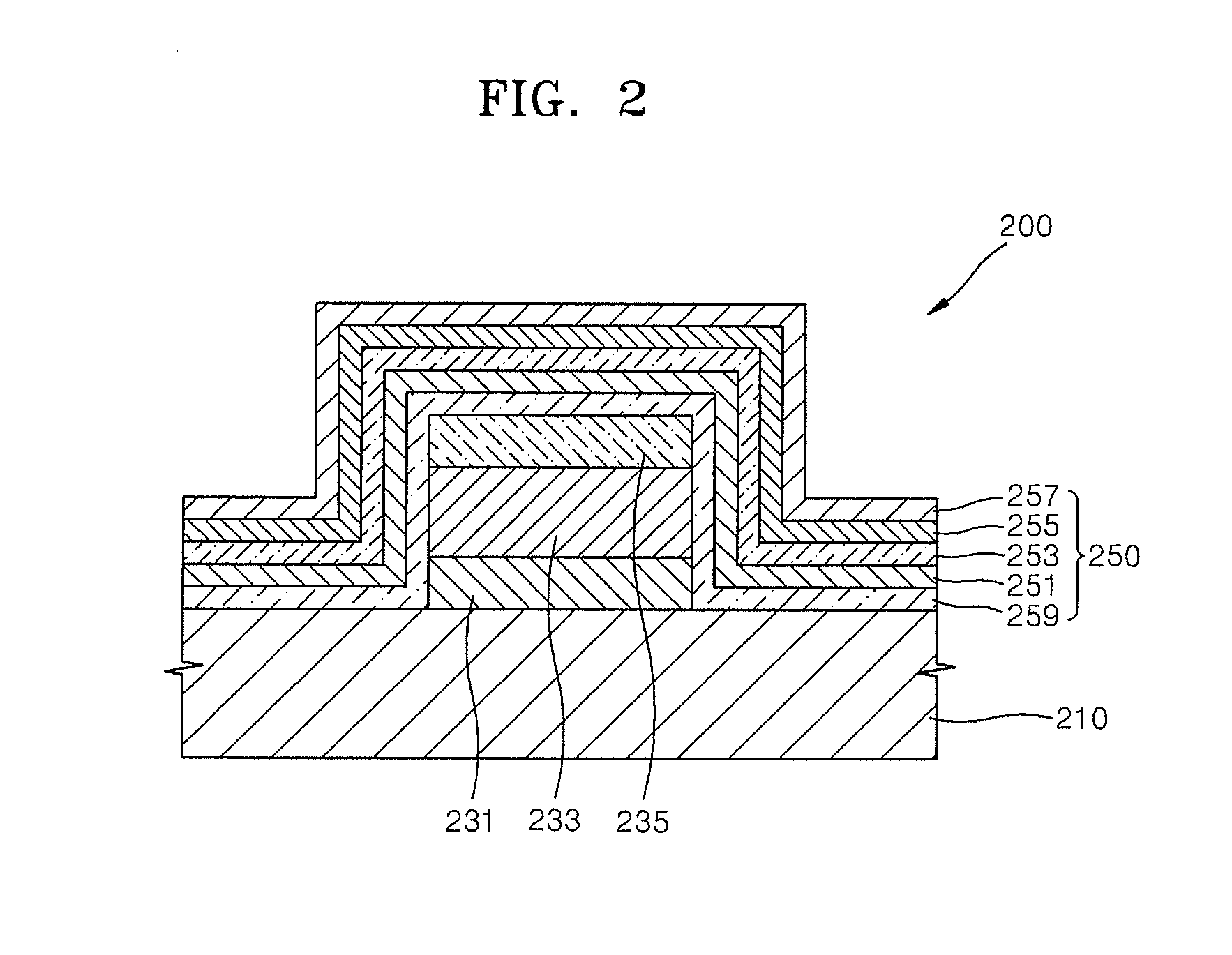
Organic light-emitting display apparatus having a hydroxyquinoline-based layer as part of the sealing structure and method of manufacturing the same
An organic light-emitting display apparatus includes: a substrate; a first electrode on the substrate; an organic emission layer on the first electrode; a second electrode on the organic emission layer; and a sealing layer including a capping film, a first inorganic film, a first organic film, a second inorganic film and a hydroxyquinoline-based blocking film between the second electrode and the first organic film that are sequentially stacked on the second electrode, the hydroxyquinoline-based blocking film comprising a hydroxyquinoline-based compound.
Owner:SAMSUNG DISPLAY CO LTD
Lithium metal quinolates and process for preparation thereof as good emitting, interface materials as well as n-type dopent for organic electronic devices
ActiveUS20130015431A1Minimize timeOrganic chemistryElectroluminescent light sourcesElectron injectionLithium metal
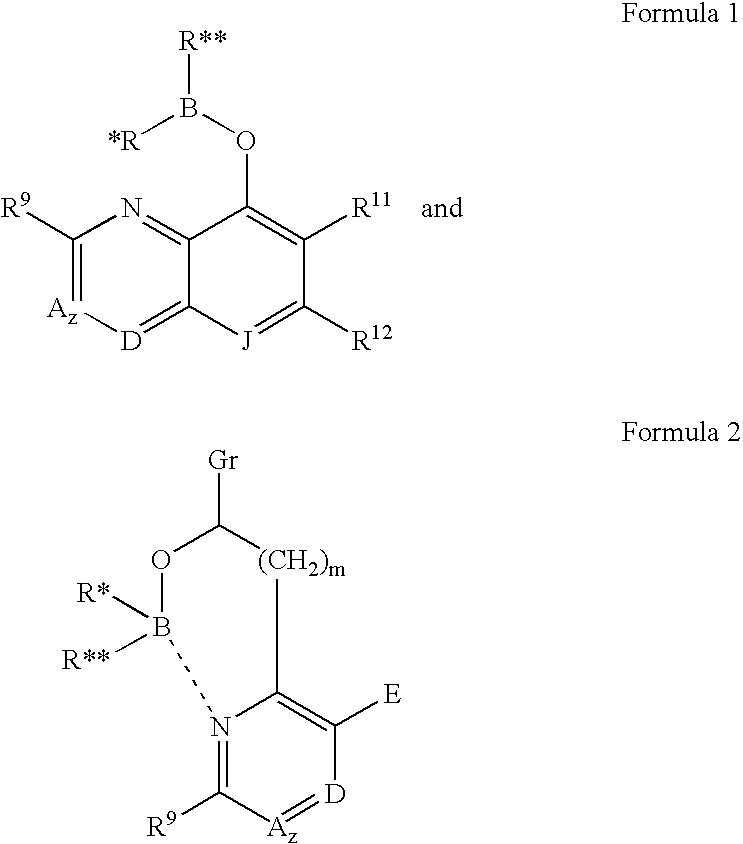
Invention relates to a single step preparation of alkali metal quinolate of general formula 1whereinM=Lithium, sodium or potassium;R═H, alkyl (C1-C6), alkoxy, aryl, aryloxy, amino, amido or halogen (Cl, F, Br, I) which is substituted or unsubstituted with direct reaction of metal with 8-hydroxyquinoline. Substituted 8-hydroxyquinoline optionally have at least one substituent selected from the group consisting of alkyl, alkoxy, aryl, aryloxy, amino, amido at 2, 5 or 7 position. Halogen substituted 8-hydroxyquinolates are also prepared from this method in the yield of 90-95% from polar solvents like acetonitrile. These complexes are useful as light emitting and electron injecting materials in organic electronic devices. Also the doping of these materials in electron transport materials improves their electron mobility.
Owner:COUNCIL OF SCI & IND RES
Tabletting method for locust stem tip chromosome
InactiveCN101482515AEasy to operateGood repeatabilityPreparing sample for investigationMaterial analysis by optical meansNitraria billardiereiMetaphase chromosome
The invention provides a robina stem-apex chromosome pressing method, comprising: taking material, preprocessing, fixing, dissociating, pressing, dyeing, striking, microscopic examining, characterized in that: taking the stem-apex of robina tissue culture seedling in vigorous growth stage at 3-5pm at the culture-room temperature 22-25 DEG C., wherein the cell of the stem-apex of robina tissue culture seedling is at vigorous division stage; preprocessing the stem-apex using 8-hydroxy quinoline 0.002-0.004m for 3-5 hours; pressing the stem-apex using a cross pressing method; dyeing the pressed stem-apex using carbol fuchsin; striking the material using a pencil head and uniformly dispersing the cells to the best. The robina stem-apex chromosome pressing method has features of simple operation, high pressing efficiency, saved medicine consumption, uniform chromosome dispersion, easy counting, very easy observation of metaphase chromosome, uniform and clear dyeing, especially suitable for chromosome pressing of tough wood material.
Owner:天津市林业果树研究所
Water-rich stripping and cleaning formulation and method for using same
ActiveUS20130296215A1Photosensitive material processingInorganic non-surface-active detergent compositionsResistHydroxylamine
Owner:VERSUM MATERIALS US LLC
Novel immobilized 8-hydroxyquinoline type chelate adsorption material and preparation method thereof
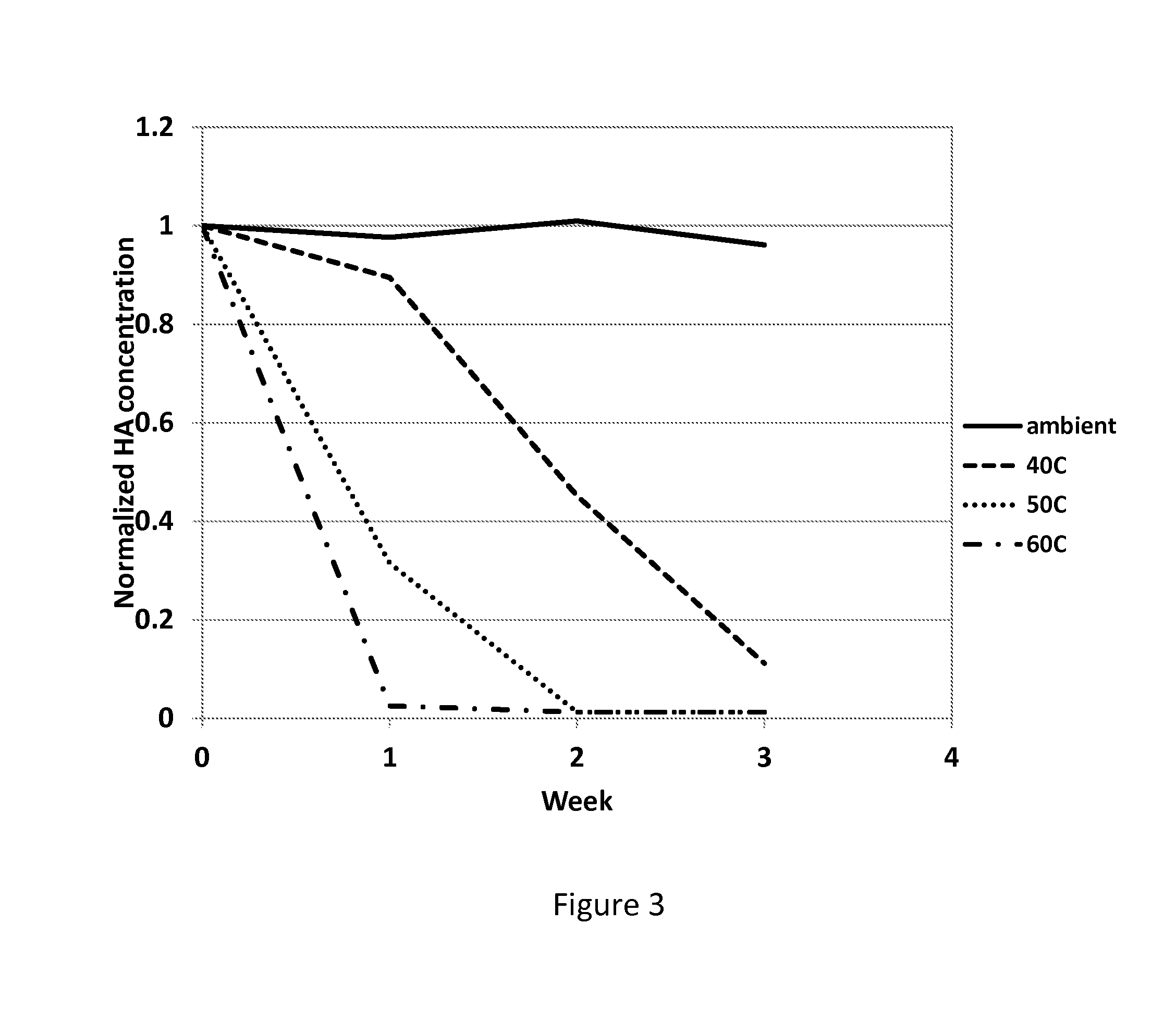
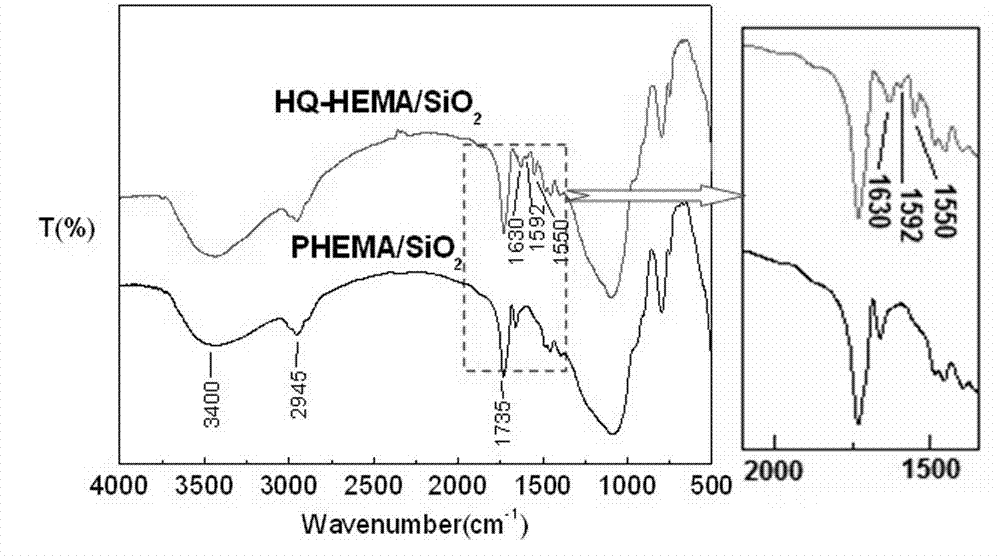
InactiveCN102773082AImprove the coordination effectHigh bonding capacityOther chemical processesWater/sewage treatment by sorption(Hydroxyethyl)methacrylateIndustrial waste water
The invention belongs to the field of chelate resin materials, and particularly relates to a novel immobilized 8-hydroxyquinoline type chelate adsorption material and a preparation method thereof. 0.95-1.85mmol / g of ligand 8-hydroxyquinoline is bonded to the surface of a silica gel micro-particle modified by hydroxyethyl methacrylate (HEMA). The preparation method includes the steps: grafting the hydroxyethyl methacrylate on the surface of the silica gel micro-particle modified by amino containing silane coupling agents; and fixing the ligand 8-hydroxyquinoline on the surface of the silica gel micro-particle modified by polymer. The material is large in 8-hydroxyquinoline bonding quantity, simple in synthesis process, moderate in condition, short in time, easy to control and fine in mechanical property, is repeatedly used, has a high coordinated complexing capacity for metal ions, can enrich and recycle various precious metal ions, particularly has excellent adsorption and selection performances for heavy metals such as copper, nickel and lead, and can be used for purifying industrial wastewater.
Owner:ZHONGBEI UNIV
Nano rare earth m-hydroxy benzoic acid 8-hydroxyquinoline antibacterial agent and method for preparing same
InactiveCN1704403AImprove antibacterial propertiesDoes not affect processabilityBiocideOrganic chemistryBenzoic acidEscherichia coli
The invention relates to a process for preparing nano rare earth m-hydroxy benzoic acid 8-hydroxyquinoline antibacterial agent which comprises, mixing 0.01 mol of sodium m-hydroxybenzoate with 0.02 mol of 8-hydroxyquinoline with a magnetic stirrer to obtain mixed ligand solution, instilling the mixed ligand solution into 0.01 mol REC136 H2O at 40-70 deg. C, adjusting pH to above 6, reacting 2-6 hrs, ageing 0.5-4 hrs, filtering by suction, removing chloride ions (Cl-) through secondary distilled water washing, vacuum drying 4-10 hours.
Owner:SHANGHAI NORMAL UNIVERSITY
Sulfuric acid-boric acid-additive ternary anodizing fluid
InactiveCN101792920AImprove fatigue lifeIncrease the scope of applicationAnodisation8-HydroxyquinolineExcessive growth
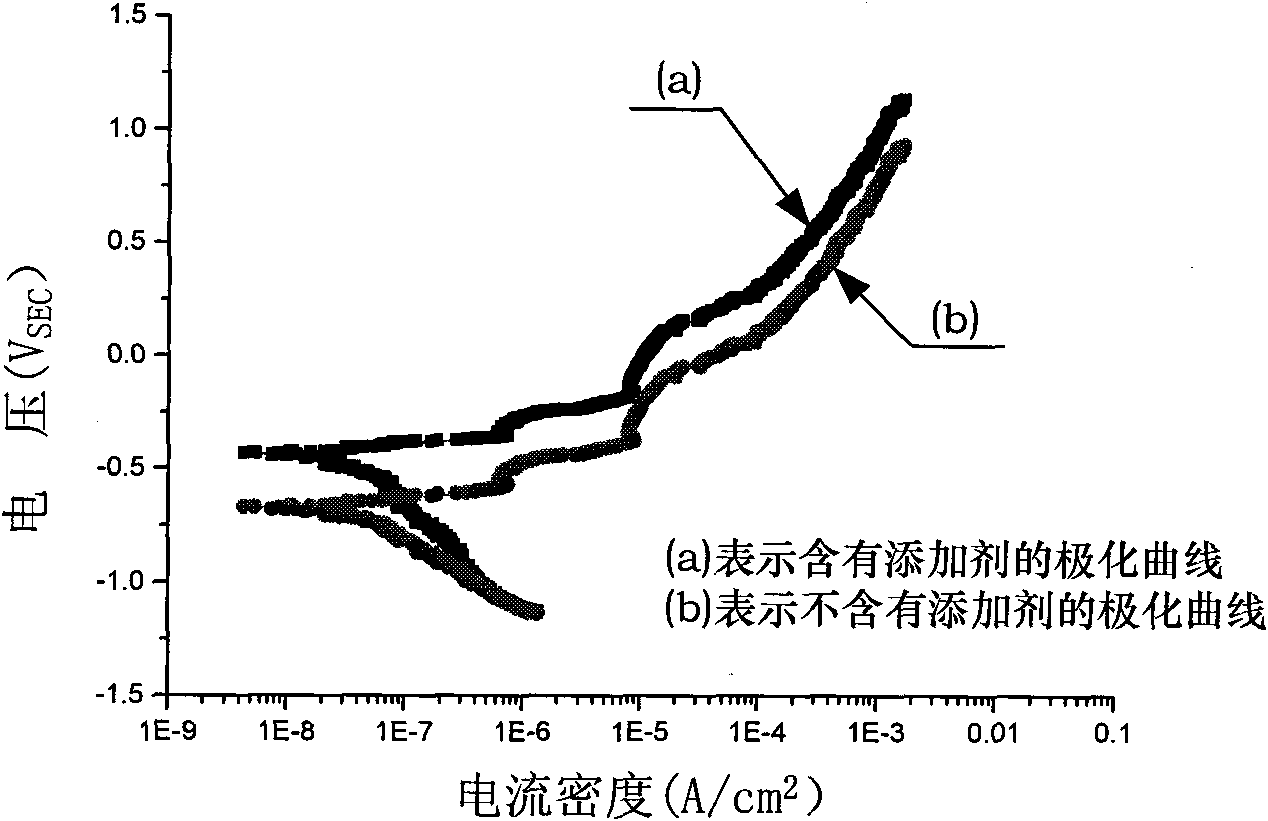
The invention discloses a sulfuric acid-boric acid-additive ternary anodizing fluid, which is prepared by adding 1.6-7.6ml of sulfuric acid, 0.1-1.1g of boric acid and 5-100mg of additive into 100ml of deionized water. The additive is benzotriazole, 2-mercaptobenzothiazole, dithizone or 8-oxyquinoline. The anodizing fluid of the invention is the improvement of aluminium and aluminium alloy sulfuric acid-boric acid binary anodizing fluid, and cupreous efficient cathodic corrosion inhibitor is added to enable that preferential adsorption of the corrosion inhibitor is carried out on the copper-containing particle position of an aviation aluminum alloy surface, thus forming a compact reticulatepattern complexing product, providing targeted protection on copper-containing aluminum alloy phase particles, decreasing the current concentration tendency, and inhibiting excessive growth of oxidation film cavern defect at the position of the copper-containing aluminum alloy phase particles.
Owner:BEIHANG UNIV
Derivatives of 1,2-dihydro-7-hydroxyquinolines containing fused rings
The present invention describes novel dyes, including coumarins, rhodamines, and rhodols that incorporate additional fused aromatic rings. The dyes of the invention absorb at a longer wavelength than structurally similar dyes that do not possess the fused aromatic rings. Many of the dyes of the invention are useful fluorescent dyes. The invention includes chemically reactive dyes, dye-conjugates, and the use of such dyes in staining samples and detecting ligands or other analytes.
Owner:MOLECULAR PROBES
Quinolyl amide derivative and its prepn process and use
The present invention relates to quinolyl amide derivatives, their preparation process, medicine compositions and use in preparing medicines for treating and / or preventing chronic nephritis, rheumarthritis, insulin dependent diabetes mellitus, systemic lupus erythematous, multiple sclerosis and other diseases.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
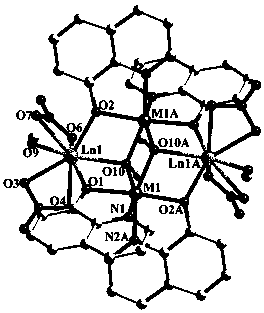
Rare earth (III)-transition (II) mixed metal fluorescence complex and preparing method and application thereof
InactiveCN105503965AHigh yieldEnhanced fluorescence emission intensityGroup 8/9/10/18 element organic compoundsOrganic chemistry methodsFluorescenceSolvent
The invention relates to a preparing method of a rare earth (III)-transition (II) mixed metal fluorescence complex constructed by means of 8-hydroxyquinoline ligand and the application of the complex in fluorescence probes. The chemical formula of the complex is [Ln2M2(CH3OH)2(eq)4(NO3)4(CH3O)2], wherein Ln is plus trivalence lanthanide rare earth ion Dy(III) or Tb(III), M is plus bivalence transition metal ion Co(II) or Ni(II), eq is minus univalency negative ion of 8-hydroxyquinoline, and CH3O is deprotonated methyl alcohol negative ion. The complex is prepared by means of the solvothermal method, the yield is high, and fluorescence emission intensity is high. The mixed metal complex can recognize copper (II) ions or iron (III) ions in a highly selective mode in weak solution, and therefore the mixed metal complex can be applied to positive ion recognition and detection as a fluorescence probe compound.
Owner:TIANJIN NORMAL UNIVERSITY
Blocked high-temperature resistant integrated solvent-free polyurethane resin for leather, and preparation method and application thereof
InactiveCN108570137AStrong production operabilityLow costPolyurea/polyurethane coatingsTextiles and paperPolyesterFoaming agent
Blocked high-temperature resistant integrated solvent-free polyurethane resin for leather, and a preparation method and application thereof are disclosed. The polyurethane resin comprises a resin component A and a resin component B. The resin component A comprises a polyether ester polyol-polyisocyanate prepolymer, polyester polyol, a small molecule alcohol chain extender, a foaming agent and a catalyst. The resin component B comprises diisocyanate, polyester polyol and a NCO blocking agent. The blocking agent is one or more selected from phenol, nitrophenol, 2-pyridinol, 3-hydroxyquinoline, triphenylmethanethiol, hexanethiol, dodecanethiol, 3,5-dimethylpyrazole, 1,2,4-triazole, 2-methyl imidazoline, 2-ethyl-4-methylimidazole, caprolactam, N-methylacetamide, phenylacetamide, bisulfate or amine. The polyurethane resin can be used for preparing high-temperature resistance solvent-free polyurethane synthetic leather. The production efficiency is high. Equipment specially used for solvent-free leather synthesis is not needed, operable time is long after product mixing and stability is high.
Owner:ZHEJIANG HUAFON SYNTHETIC RESIN
Organic monomer containing 8-hydroxyquinoline boron, conjugated polymer based on monomer, preparation method and application
ActiveCN106317383AImprove solubilityImprove film formationGroup 3/13 element organic compoundsColor/spectral properties measurementsSolubilityQuantum yield
The invention discloses an organic monomer containing 8-hydroxyquinoline boron, a conjugated polymer based on the monomer, a preparation method and application, and belongs to the technical field of preparation of the conjugated polymer containing a main group element and application of photoelectrical properties of the conjugated polymer. The preparation method of the compound has the outstanding advantages of being easy and convenient to operate, mild in reaction condition, capable of conducting large-scale production, high in adaptability to substrate, free of column chromatography purification and the like; the prepared monomer and the polymer are good in photochemical stability, high in fluorescence quantum yield and excellent in solubility, and a firm foundation is laid for sensing application and photoelectric property application of the monomer and the polymer.
Owner:SHAANXI NORMAL UNIV
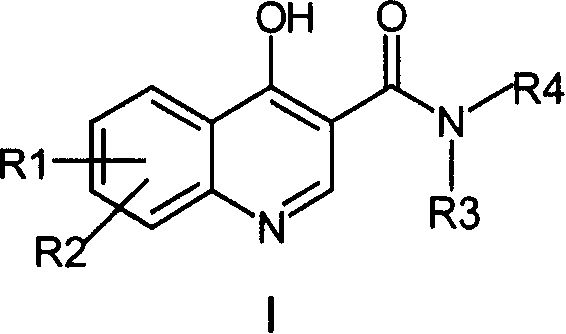
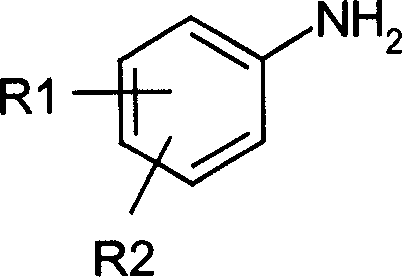
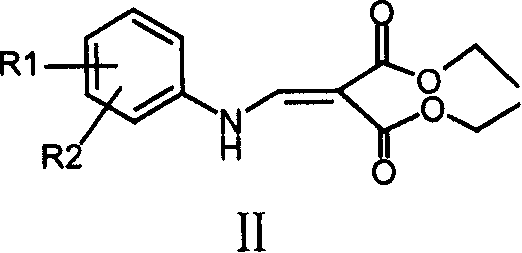
4-Hydroxyquinoline-3-carboxamides and hydrazides as antiviral agents
The present invention provide 4-hydroxyquinoline-3-carboxamide and hydrazide compounds of formula (I). These compounds are useful to treat or prevent the herpesviral infections, particularly, human cytomegaloviral infection.
Owner:PHARMACIA & UPJOHN CO
Olefin monomer containing 8-hydroxyquinoline metal complex and its production method and use
InactiveCN1569837AGood solubilityImprove stabilityGroup 1/11 element organic compoundsGroup 3/13 element organic compoundsIonChemistry
The invention relates to a series of olefin monomer of a polymerisable functional group 8-hydroxyquinoline metal complex, its preparation process and use thereof, wherein the preparation process consists of synthesizing alkene monomers containing 8-hydroxyquinoline through reaction between vinyl compound containing reacting function group with modified 8-hydroxyquinoline, then compounding with metallic ions and 8-hydroxyquinoline.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com