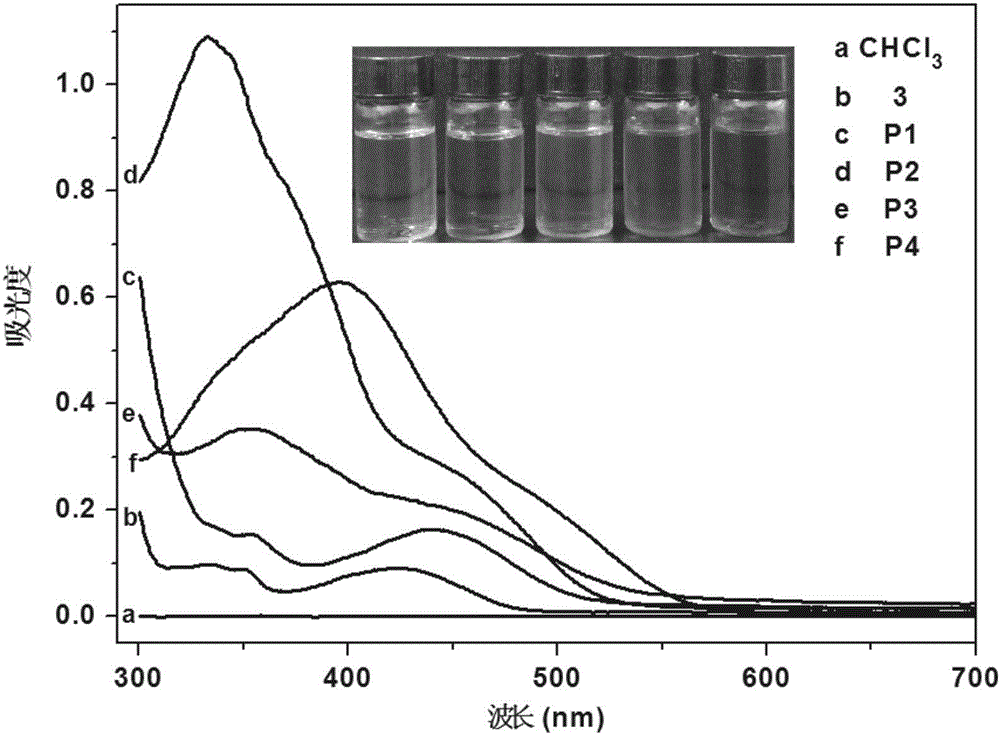
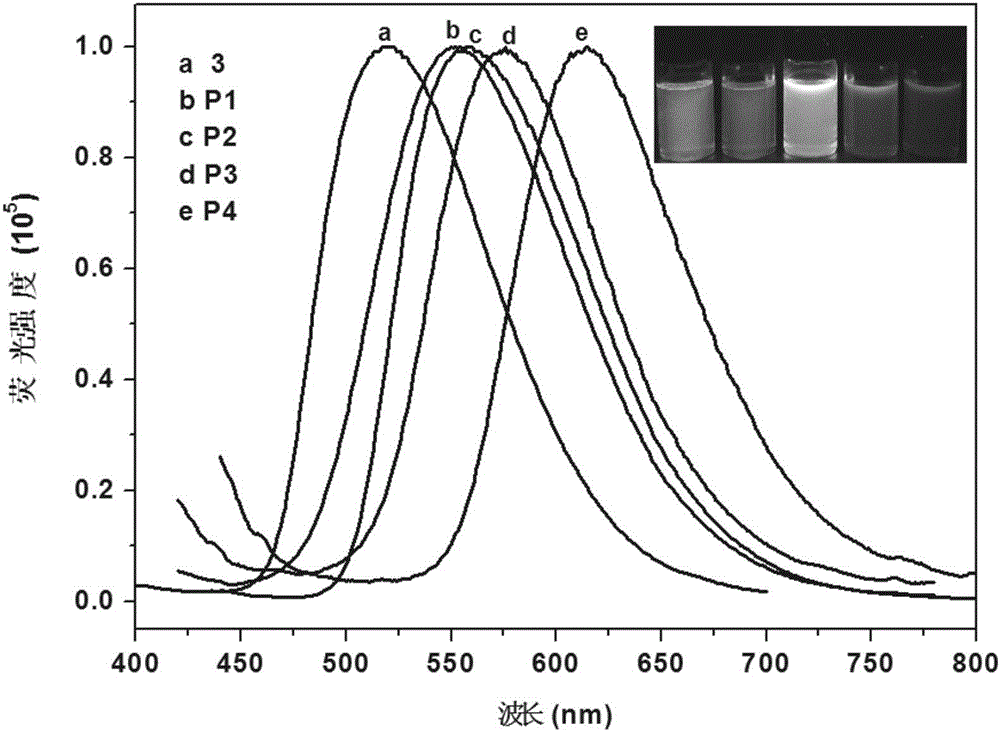
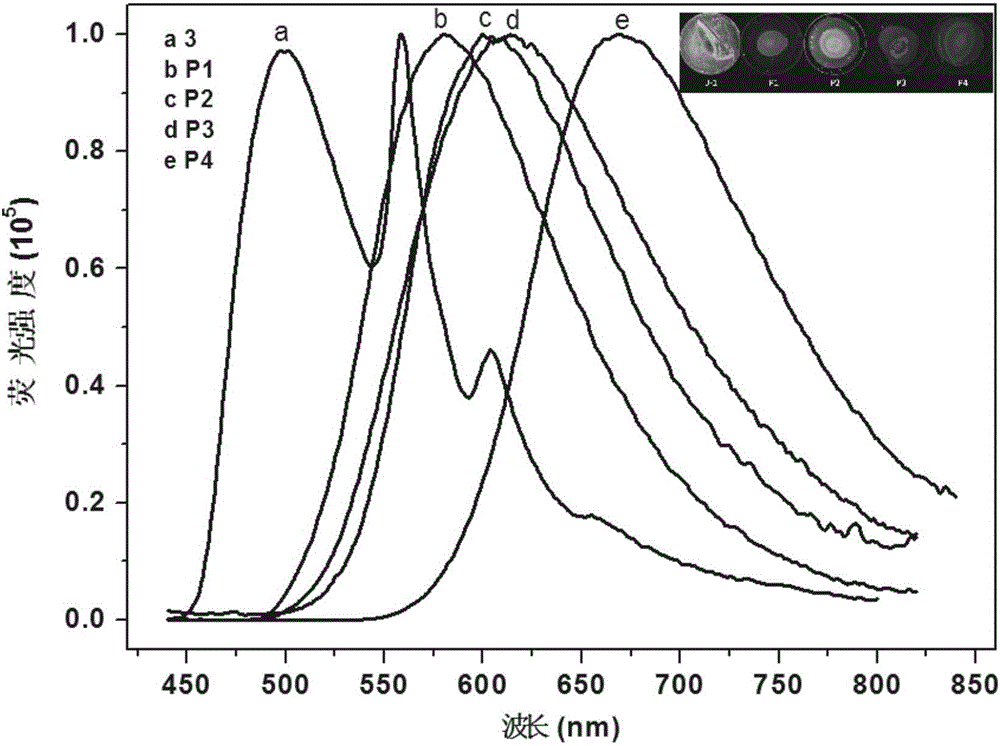
Organic monomer containing 8-hydroxyquinoline boron, conjugated polymer based on monomer, preparation method and application
A technology of hydroxyquinoline boron and conjugated polymers, which is applied in the fields of compounds containing elements of Group 3/13 of the periodic table, organic chemistry, chemical instruments and methods, etc., and can solve problems such as limiting the wide application of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0104] 2. Preparation of fluorescent film
[0105] The resulting compound was dissolved in chloroform to prepare a 1-10 mg / mL stock solution. Each time, 20-50 μL of the stock solution was dropped and coated on the surface of a specially treated glass substrate to prepare a fluorescent film.
[0106] Preferably, in the step of preparing monomer and polymer in the present invention:
[0107] In the step 1) of synthesizing compound 1, the optimal molar ratio of the described diisopropylamine, borane dimethyl sulfide, n-butyllithium, trimethylchlorosilane and tetrahydrofuran is: 1:1:1:1: 4.4;
[0108] In step 2) of synthesizing compound 2, the optimal molar ratio of compound 1, 1-(4-alkyl)bromobenzene, magnesium, hydrochloric acid and tetrahydrofuran is: 1:3:3.3:15:98.5;
[0109] In the step 3) of synthesizing compound 3, the optimal molar ratio of compound 2, 5,7-diiodo-8-hydroxyquinoline and ether is 1:1:57.8;
[0110] In the step 4) of the synthetic compound 4, the alkyl su...
Embodiment 1
[0119] Preparation of an organic monomer and four conjugated polymers
[0120] ①Synthesis of compound 1
[0121] Under an argon protective atmosphere, 7 mL of diisopropylamine was added to 18 mL of anhydrous tetrahydrofuran solution and fully stirred, and under ice bath conditions, 5 mL of borane dimethyl sulfide in tetrahydrofuran (10 mol / L) was added dropwise In the above reaction solution, after the dropwise addition, the reaction was continued for 1.5 hours under ice-bath conditions; Stir for 2 hours; finally, add 6.4 mL of trimethylchlorosilane dropwise to the reaction system, and immediately produce a large amount of white precipitates, and stir at room temperature for 2 hours after all the addition. After the reaction, the reaction solution was left to stand for 1 hour and filtered under the protection of argon to obtain a colorless and transparent filtrate which was compound 1, which was stored under the protection of argon for future use, with a yield of about 99%. ...
Embodiment 2
[0143] In this example, in step ① of the synthesis of compound 1, 7 mL of diisopropylamine was added to 32.5 mL of anhydrous tetrahydrofuran solution under an argon protective atmosphere and fully stirred, and 5 mL of diisopropylamine was added dropwise under ice bath conditions The tetrahydrofuran solution (10mol / L) of borane dimethyl sulfide was added to the above reaction solution. After the dropwise addition, the reaction was continued for 1.5 hours under ice bath conditions; then 20 mL of n-butyl lithium was added dropwise to the reaction system n-Hexane solution (2.5mol / L), continue to stir for 2 hours under ice-bath conditions; finally, add 6.4mL trimethylchlorosilane dropwise to the reaction system, a large amount of white precipitate will be produced immediately, after all the addition, stir at room temperature 2 hours. The molar ratio of diisopropylamine, borane dimethyl sulfide, n-butyllithium, trimethylchlorosilane and tetrahydrofuran is 1:1:1:1:5.5. After the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com