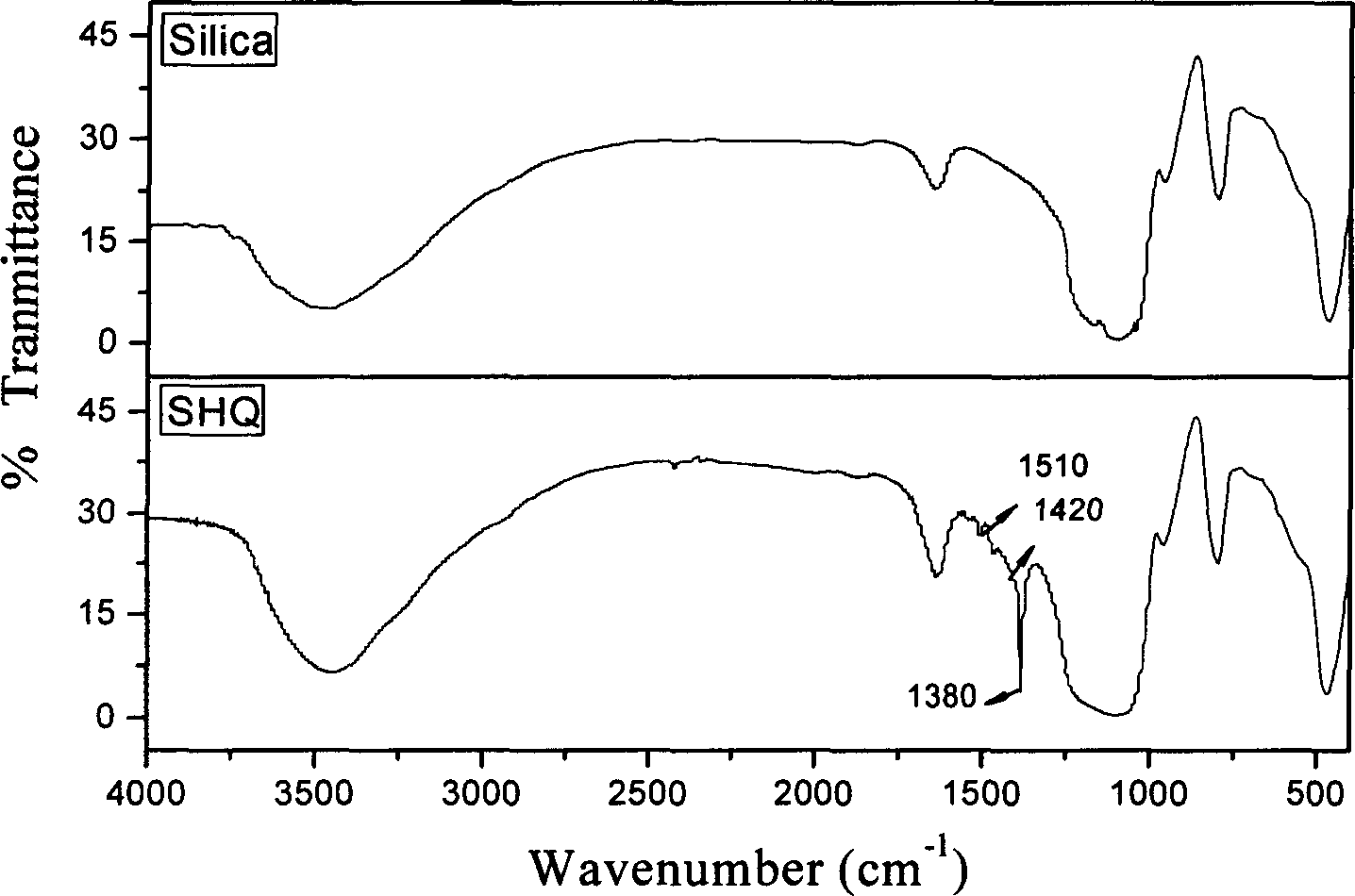
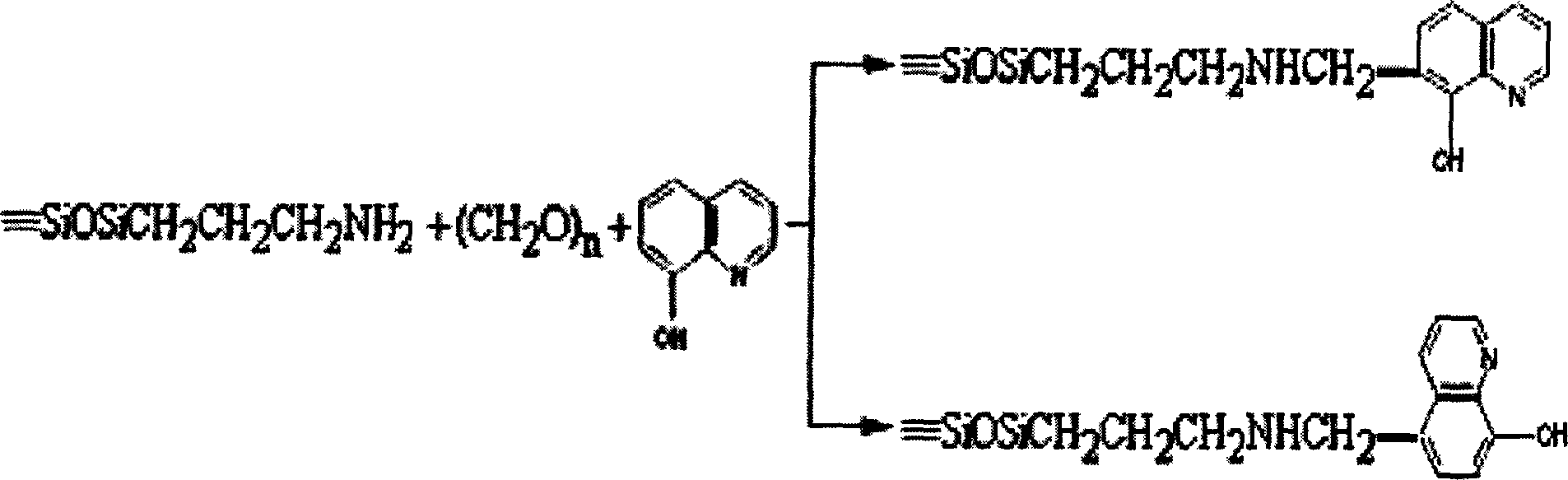
8-hydroxy quinoline type chelated resin and its synthesis
A hydroxyquinoline-type, chelating resin technology, applied in chemical instruments and methods, chelate ion exchange, ion exchange, etc., can solve the problems of low 8-hydroxyquinoline bonding and complex synthesis methods, etc. Achieve the effects of easy control of the synthesis process, simple synthesis process, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Weigh 10 g of commercially available 50-100 mesh chromatography silica gel and place it in a 100 ml round bottom flask, add 0.1 mol / L superior-grade pure HCl solution to it, let stand at 90°C for 24 hours, and wash with deionized water To neutral, dry in a vacuum oven at 150°C;
[0028] (2) Put the silica gel treated in step (1) into a 100ml round-bottomed flask, add 50mL of toluene and 5mL of γ-aminopropyltriethoxysilane that have been re-distilled and calcium chloride dehydrated, stir and reflux for 18h, and use Toluene, acetone, ethanol to wash off excess silylating reagent, dry at 120°C for later use;
[0029] (3) Weigh 0.73g of 8-HQ, put it in a 100ml round bottom flask and dissolve it in 25ml of ethanol under slight heat, then gradually add 0.3g of paraformaldehyde and the product treated in step (2) successively, at a constant temperature of 50°C Stirring and reacting for 7 hours;
[0030] (4) The product treated in step (3) was dried at 100° C. for 1 h, wa...
Embodiment 2
[0037] All steps are the same as in Example 1, except that the silylating agent used is changed to γ-aminopropyltrimethoxysilane. That is to make 8-hydroxyquinoline type chelating resin.
Embodiment 3
[0039]All the steps are the same as in Example 1, except that the silylating agent used is changed to γ-aminopropyltrimethoxysilane; and then 1 g of 8-hydroxyquinoline-5-sulfonic acid is used for the synthesis reaction. That is to make 8-hydroxyquinoline type chelating resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com