Rare earth (III)-transition (II) mixed metal fluorescence complex and preparing method and application thereof
A transition metal ion, mixed metal technology, applied in fluorescence/phosphorescence, organic chemistry methods, chemical instruments and methods, etc., can solve problems to be further studied, and achieve high yield, high fluorescence emission intensity, selectivity and sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
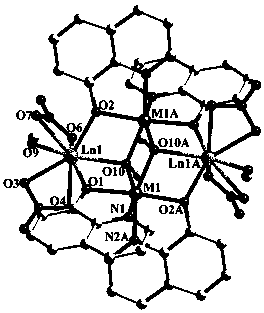
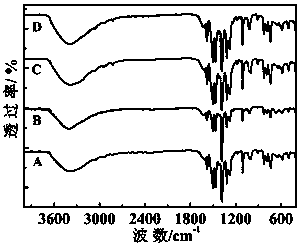
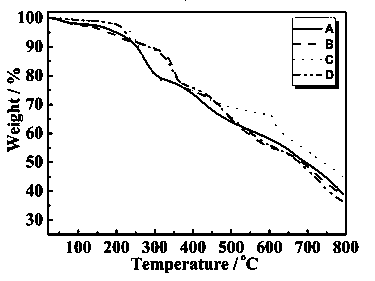
[0027] 8-hydroxyquinoline as ligand {Dy 2 co 2} Synthesis of fluorescent complex A:
[0028] 8-Hydroxyquinoline (0.3 mmol, 43.5 mg), cobalt nitrate hexahydrate (0.2 mmol, 58.2 mg), and dysprosium nitrate hexahydrate (0.2 mmol, 91.3 mg) were dissolved in anhydrous methanol (15.0 mL) , with NaHCO 3 The pH of the reaction mixture was adjusted to 5 by the solid, and it was sealed in a hydrothermal kettle after stirring for several minutes. Water heating kettle at 120 o After three days of incubation at C, at 4.0°C·h –1 The temperature was lowered to room temperature by the rate program, and the red blocky crystals were obtained, which were then washed with anhydrous methanol and dried in air.
Embodiment 2
[0030] {Dy based on 8-hydroxyquinoline as a ligand 2 Ni 2} Synthesis of fluorescent complex B:
[0031] 8-Hydroxyquinoline (0.3 mmol, 43.5 mg), nickel nitrate hexahydrate (0.2 mmol, 58.2 mg), and dysprosium nitrate hexahydrate (0.2 mmol, 91.3 mg) were dissolved in anhydrous methanol (15.0 mL) , with NaHCO 3 The pH of the reaction mixture was adjusted to 5 by the solid, and after stirring for several minutes, it was sealed in a hydrothermal kettle. Water heating kettle at 140 o After 3 days of incubation at C, at 3.2°C·h –1 The rate program cooled down to room temperature to obtain green blocky crystals, which were then washed with anhydrous methanol and dried in air.
Embodiment 3
[0033] {Tb based on 8-hydroxyquinoline ligand 2 co 2} Synthesis of fluorescent complex C:
[0034] 8-Hydroxyquinoline (0.3 mmol, 43.5 mg), cobalt nitrate hexahydrate (0.2 mmol, 58.2 mg) and terbium nitrate hexahydrate (0.2 mmol, 90.6 mg) were dissolved in anhydrous methanol (15.0 mL), with NaHCO 3 The pH of the reaction mixture was adjusted to 6 by the solid, and it was sealed in a hydrothermal kettle after stirring for several minutes. Water heating kettle at 120 o After three days of incubation at C, at 4.0°C·h –1 The rate program cooled down to room temperature to obtain red blocky crystals, which were then washed with anhydrous methanol and dried in air.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com