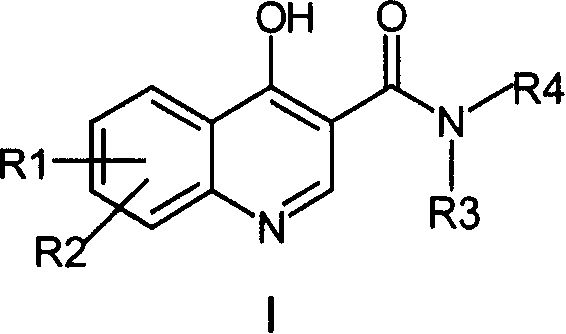
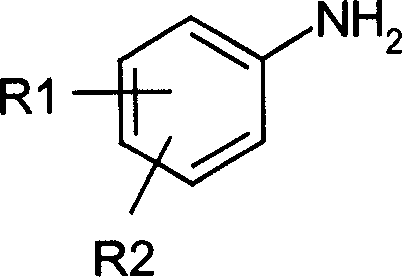
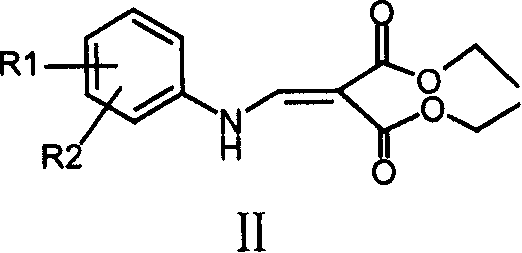
Quinolyl amide derivative and its prepn process and use
A quinoline and amide technology, applied in the field of 4-hydroxy-quinoline-3-amide compounds, can solve problems such as treating symptoms but not root causes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Embodiment 1: the synthesis of N-(3-chlorophenyl)-4-hydroxyl-7-chloro-quinoline-3-amide
[0192] 1.1 Synthesis of 3-chloroanilinodiethylmethylenemalonate: Mix 7.00 grams (0.055mol) of m-chloroaniline and 12.00 grams (0.056mol) of ethoxydiethylmethylenemalonate, add 50ml of toluene , heated to about 100°C, reacted for 5 hours, and evaporated the solvent under reduced pressure to obtain a white wax, white needle-like crystals recrystallized from petroleum ether, weighing 14.20 g, m.p.56-57°C, yield 87.0%.
[0193] 1.2 Synthesis of ethyl 4-hydroxy-7-chloro-quinoline-3-carboxylate: 14.20 grams (0.048mol) of diethyl 3-chloroanilinomethylidenemalonate was dissolved in 100ml of diphenyl ether and heated to React for 0.5 hours at about 250°C, stop heating, cool to room temperature, precipitate solid, filter the solid, wash with petroleum ether, absolute ethanol, and anhydrous ether to obtain a white solid weighing 10.50 g, m.p.>300°C, collected The rate is 87.5%.
[0194] 1.3...
Embodiment 2
[0195] Example 2: N-methyl-N-phenyl-4-hydroxyl-7-chloro-quinoline-3-amide
[0196] The compound was prepared according to the method 1.3 in Example 1, and the substituted amine used was N-methylaniline. m.p.276-278°C, yield 41.1%. 1 HNMR (DMSO-d 6 , δ): 11.91 (s, 1H, OH), 8.04 (d, 1H, J=5.9Hz), 7.96 (d, 1H, J=8.8Hz), 7.51 (d, 1H, J=1.7Hz), 7.20 -7.30 (m, 5H), 7.10 (t, 1H, J=7.0Hz), 3.37 (s, 3H).
Embodiment 3
[0197] Example 3: N-phenyl-4-hydroxy-7-chloro-quinoline-3-amide
[0198] The compound was prepared according to the method 1.3 in Example 1, and the amine used was aniline. m.p.>300°C, yield 88.7%. 1 HNMR (DMSO-d 6 , δ): 12.89(S, 1H), 12.29(S, 1H), 8.86(S, 1H), 8.34(d, 1H, J=8.7Hz), 7.3-7.8(m, 6H), 7.14(t, 1H, J=7.3Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com