4-Hydroxyquinoline-3-carboxamides and hydrazides as antiviral agents
A compound and alkyl technology, which can be applied to antiviral agents, compounds of group 4/14 elements of the periodic table, phosphorus organic compounds, etc., can solve the problems of no references, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
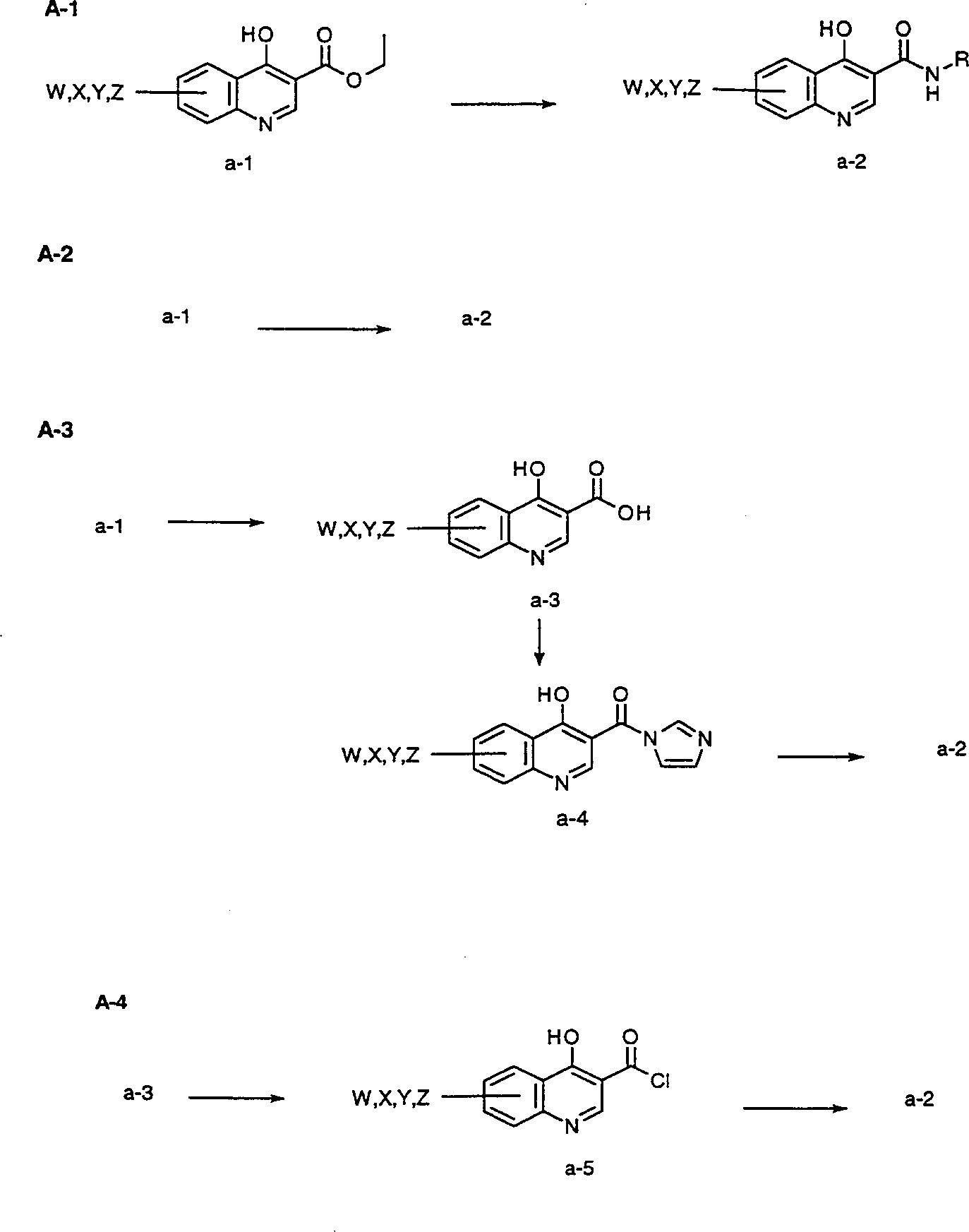
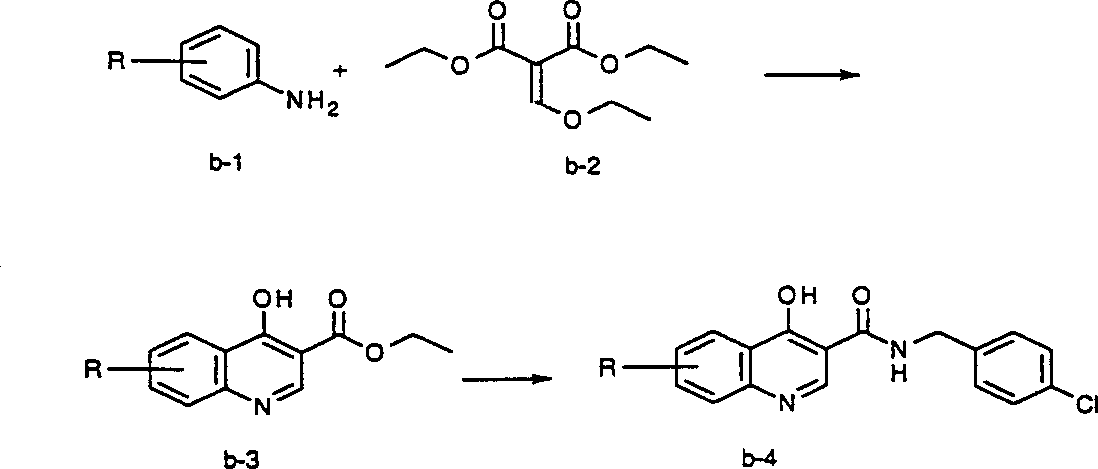
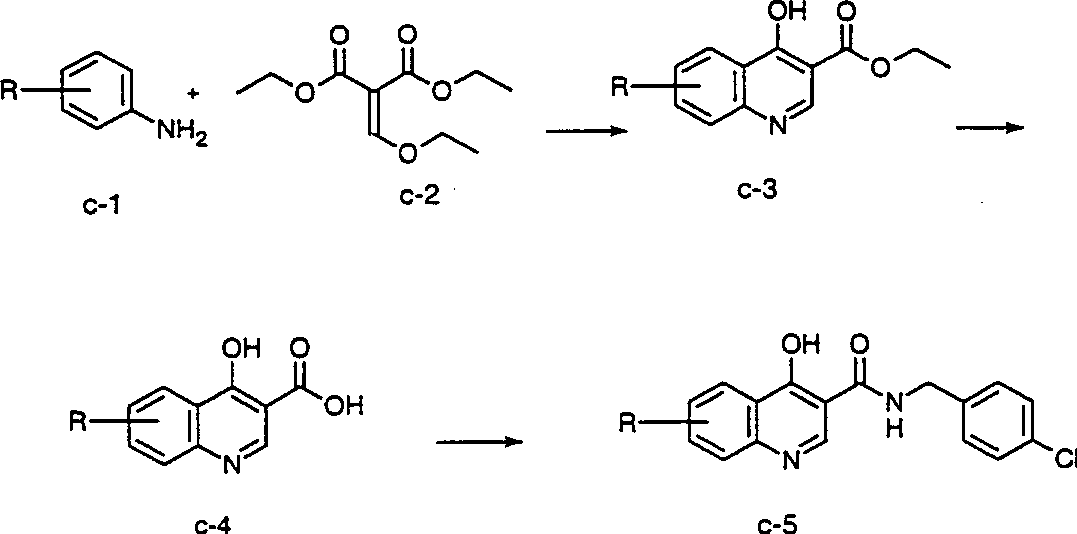
[0180] Figures A-V The preparation of compounds of the invention is described. All starting materials were prepared by methods described in these figures or by methods analogous thereto, which are well known to those of ordinary skill in the art of organic chemistry. All final compounds of the invention are prepared by methods described in these figures or by methods analogous thereto, which are well known to those of ordinary skill in the art of organic chemistry. All variables used in the figures are as defined below or in the claims.
[0181] exist Figure A Among them, the compounds of the invention can be prepared by one of several methods starting from the appropriate 4-hydroxyquinoline-3-carboxylate a-1. In the first method A-1, ester a-1 is suspended in 5-10 parts of amine and the mixture is heated to 190-200° C. for 30 minutes to 4 hours. Product a-2 was isolated by diluting the cooled reaction mixture with hexane or toluene and collecting the solid precipitate b...
Embodiment 2
[0632] Elemental analysis found values: C, 56.74; H, 3.44; N, 7.35; Cl, 9.04. Example 2 7-amino-N-[(4-chlorophenyl)methyl]-4-hydroxyl-3-quinoline carboxamide
[0633] A suspension of 0.60 mL of 4-chlorobenzylamine and 0.200 g of ethyl 4-hydroxy-7-nitro-3-quinolinecarboxylate was heated at 180° C. for 1 hour. The reaction solution was cooled to room temperature. Ethyl acetate was added and the solid formed was collected and washed with ethyl acetate to give 0.071 g of the title compound as a tan solid.
[0634] The physical properties are as follows:
[0635] Mp 244-245°C (decomposition).
[0636] 1 H NMR (DMSO) δ 12.10, 10.63, 8.44, 7.85, 7.37, 7.31, 6.67, 6.53, 6.13, 4.49.
[0637] 13 C NMR (DMSO) δ 175.9, 165.6, 153.4, 142.8, 141.9, 139.3, 131.8, 129.6, 128.8, 127.1, 116.9, 114.8, 109.8, 97.9, 41.7.
[0638] IR (grinding) 3480, 3357, 2748, 2726, 1650, 1586, 1553, 1528, 1506, 1494, 1480, 1281, 789, 737, 621cm -1 .
[0639] MS(EI) m / z 327(M + ), 329, 327, 188, 18...
Embodiment 3
[0640] HRMS (EI) found 327.0778. Example 3 N-[(4-chlorophenyl)methyl]-8-fluoro-4,6-dihydroxy-3-quinoline carboxamide
[0641] To a mixture of 10% Pd / C and 2.0 g 3-fluoro-4-nitrophenol in 10 mL tetrahydrofuran and 10 mL methanol was added 4.817 g ammonium formate. After the reaction solution was stirred at room temperature for 1 hour, it was filtered through celite and then concentrated. Then 2.57 mL of diethyl ethoxymethylene malonate and 10 mL of xylene were added to the residue. The mixture was heated to 135°C for 1 hour and the ethanol formed was removed using a Dean-Stark trap. The reaction solution was cooled. The solid formed was collected, washed with hexanes and dried. The solid was then dissolved in 50 mL of diphenyl ether and heated to 250°C for 3 hours, collecting the ethanol with a Dean-Stark trap. The solution was cooled and diluted with hexanes. The solid formed was collected and dried to yield 0.527g. A solution of 0.415 g of this solid in 4 mL of 4-chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com