Graphene oxide/hyaluronic acid nanometer drug carrier material, preparation method and application of graphene oxide/hyaluronic acid nanometer drug carrier material
A nano drug carrier, hyaluronic acid technology, applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, etc., to achieve good biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
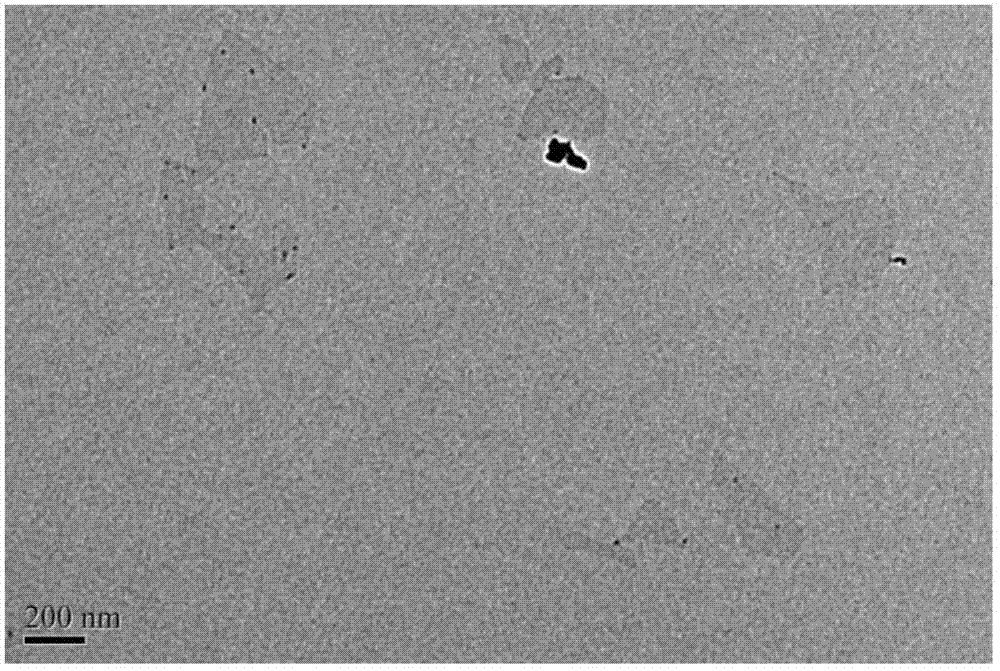
[0034] (1) Mix 2 grams of natural graphite powder and 60 grams of sodium chloride together and grind them thoroughly. After grinding for 30 minutes, dissolve them in water and filter them with suction;
[0035] (2) After drying the above filter paper, put it into a 100ml round-bottomed flask filled with 23mL of concentrated sulfuric acid, add 0.5g of sodium nitrate at the same time, place it in an ice-water bath at 6°C for magnetic stirring, and after stirring for 30min, slowly Add 3.0 g of potassium permanganate, and continue stirring in a water bath at 10°C for 8 hours;
[0036] (3) Maintain the above reaction (2) Stir at 35°C for 30 minutes, at 65°C for 45 minutes, and at 98°C for 30 minutes;
[0037] (4) After step (3), add 46mL of deionized water, continue to stir for 30min, and then add 200mL of 3% H 2 o 2 Aqueous solution; stop the reaction after stirring for 30min; then ultrasonicate the reaction solution in an ultrasonic machine for 2h;
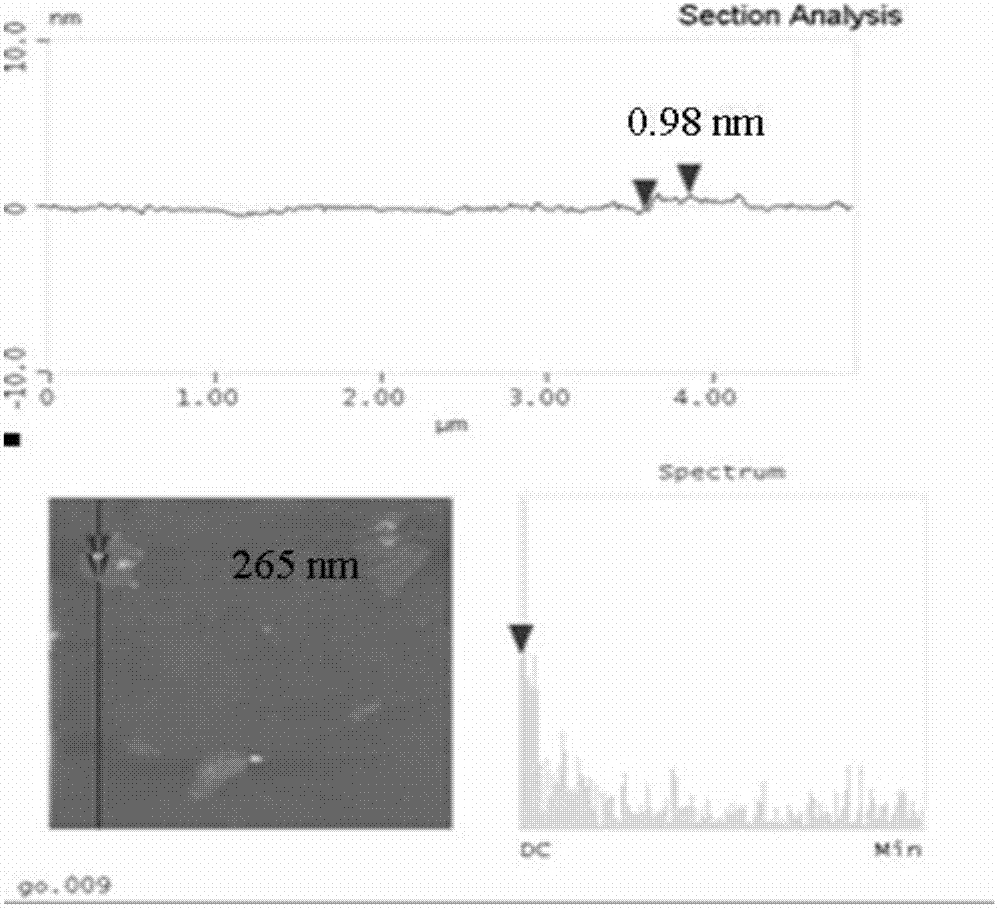
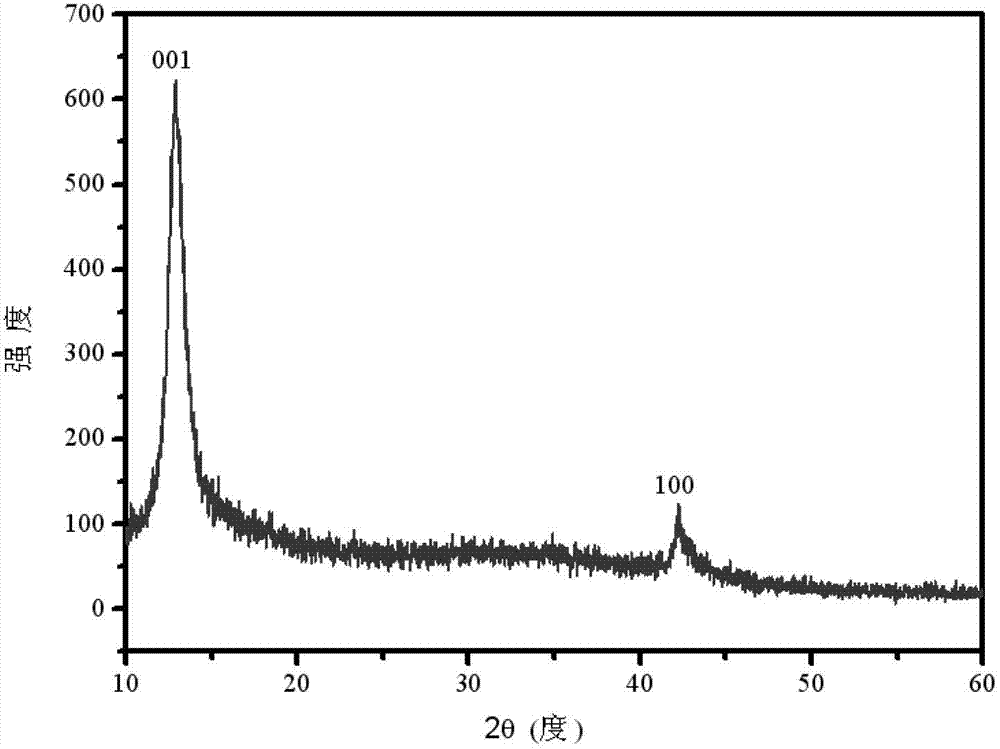
[0038] (5) The reaction so...
Embodiment 2
[0043] (1) Get the dry graphene oxide that 50mg embodiment 1 makes, be dispersed in aqueous solution according to the concentration of 1mg / mL; According to graphene oxide: EDC: the ratio of NHS mass ratio is 1:5:15 and adds EDC and NHS;
[0044] (2) Add 1 mg / mL NaOH to adjust the pH of the solution to 5.76;
[0045] (3) Add 520 mg of adipic acid hydrazide (ADH), and stir for 24 hours at room temperature;
[0046] (4) Wash several times by centrifugation with deionized water, and freeze-dry for 24 hours to obtain graphene oxide / adipate hydrazide (GO-ADH);
[0047] (5) Weigh 15mg hyaluronic acid and dissolve it in 15mL deionized water;
[0048] (6) Add EDC and NHS respectively according to the mass ratio of hyaluronic acid:EDC:NHS of 2:5:15;
[0049] (7) Use 1 mg / mL NaOH to adjust the pH of the solution to 5.6;
[0050] (8) Add 30 mg of the freeze-dried sample in step (4), and stir for 24 hours at room temperature;
[0051](9) Wash several times by centrifugation with deion...
Embodiment 3
[0055] (1) Prepare different concentrations of DOX, the solvent is DMSO, the concentrations are: 40, 60, 100, 200, 300, 1000, 1500, 2000μg·mL -1 ;
[0056] (2) Weigh 5mg of GO-HA and add to 10mL of the above solution;
[0057] (3) Place in a shaker at 200 rpm, and rotate for 20 hours at 37°C;
[0058] (4) Then wash with 3 mL of water for 3 times, and finally dilute to 25 mL with DMSO;
[0059] (5) Measure the UV absorbance of 8 supernatants with different concentrations, and calculate the drug loading respectively. The results are as follows: Figure 6 shown. It can be seen from the figure that when the concentration of doxorubicin is greater than or equal to 1000 μg·mL -1 When , the drug loading capacity of graphene oxide reaches saturation, about 300mg / g;
[0060] Figure 7 For the drug release of graphene oxide / hyaluronic acid in this example at different pH values, the solution was diluted 12.5 times with PBS before testing. (a): UV-Vis spectra of doxorubicin (DOX) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com