Self-rearranging DNA vectors
a vector and self-rearranging technology, applied in the field of dna vectors, can solve the problems of virus scale required for human clinical application, and achieve the effect of improving the persistence of the therapeutic gen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
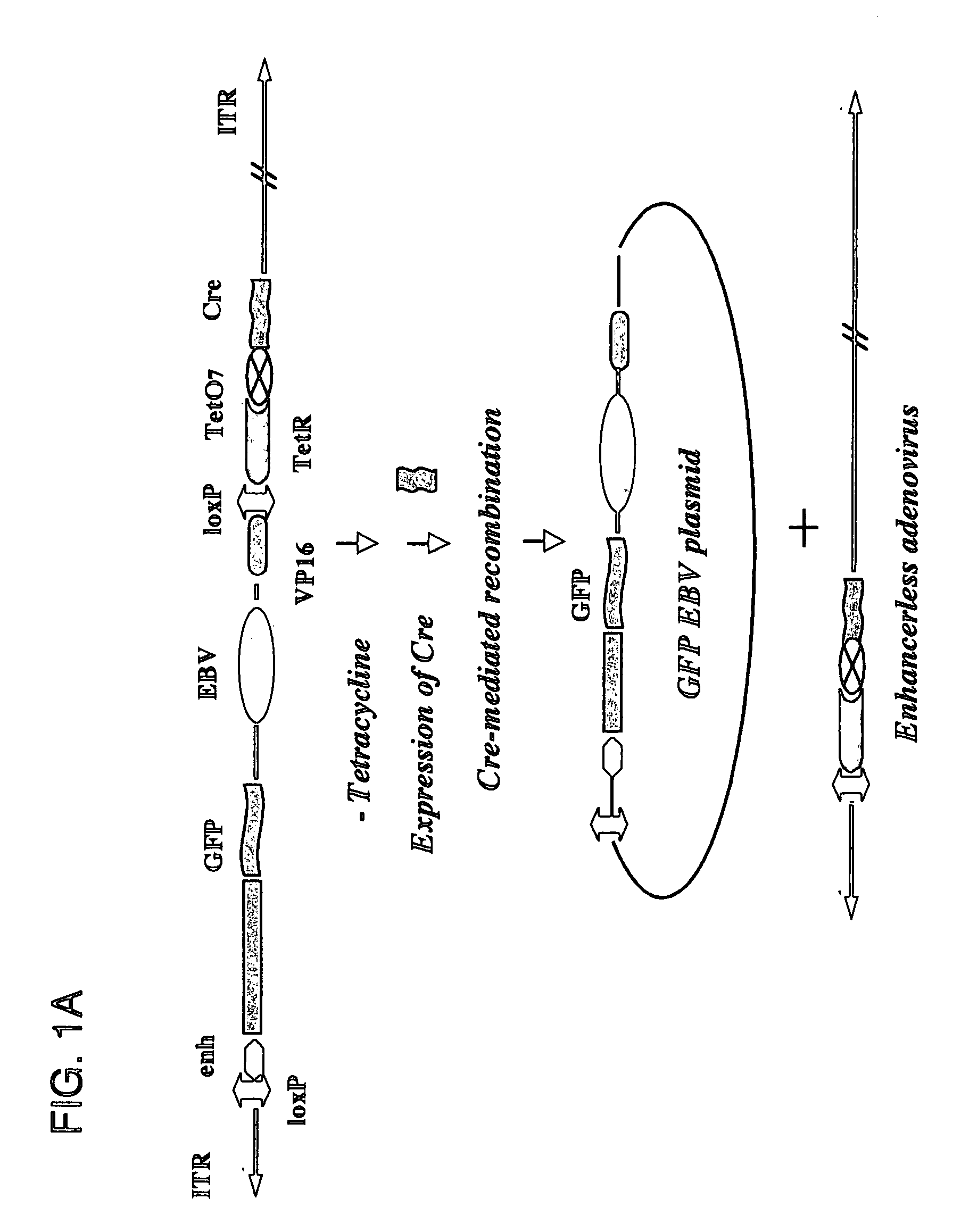
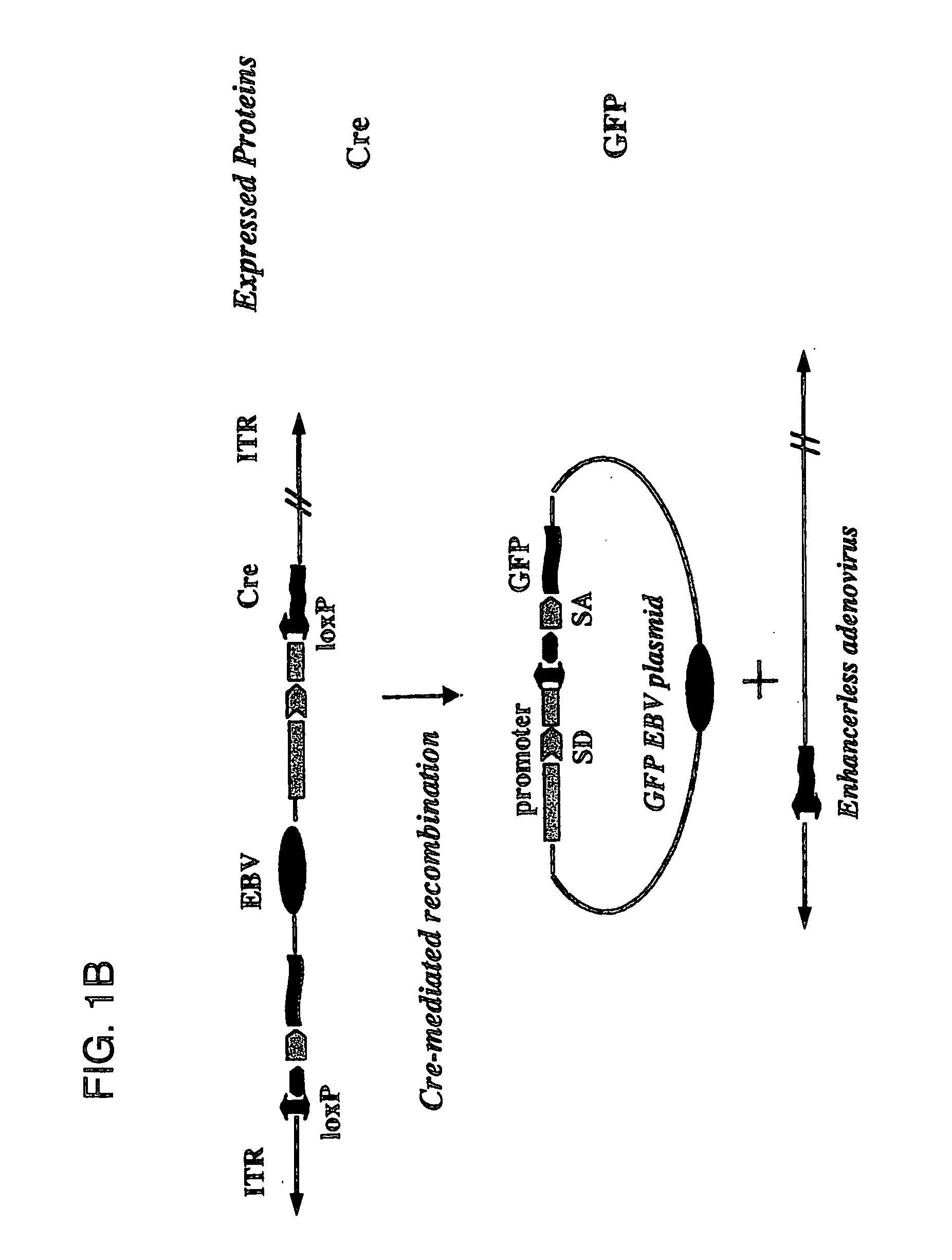
[0081] Described herein are systems for the regulated self-rearrangement of DNA vectors, for example, gene therapy vectors. Such regulated self-rearrangement has the potential to prevent unwanted expression of vector genes not required for a therapeutic effect, and to allow the stable association of the therapeutic gene with the target cell.
[0082] The essential elements of the regulated DNA rearrangement system are a gene that encodes one or more proteins that induce DNA rearrangement, a method for regulating the activity of those proteins or their abundance, and a target DNA sequence on which those proteins act. Particularly desirable are methods for regulating the activity of the proteins or their abundance which can be easily carried out on an intact organism, such as administration or withdrawal of a drug, hormone, or environmental stimulus such as heat or irradiation, which induces the activity or abundance of the proteins which cause DNA rearrangement.
[0083] Especially desir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission wavelengths | aaaaa | aaaaa |
| emission wavelengths | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com