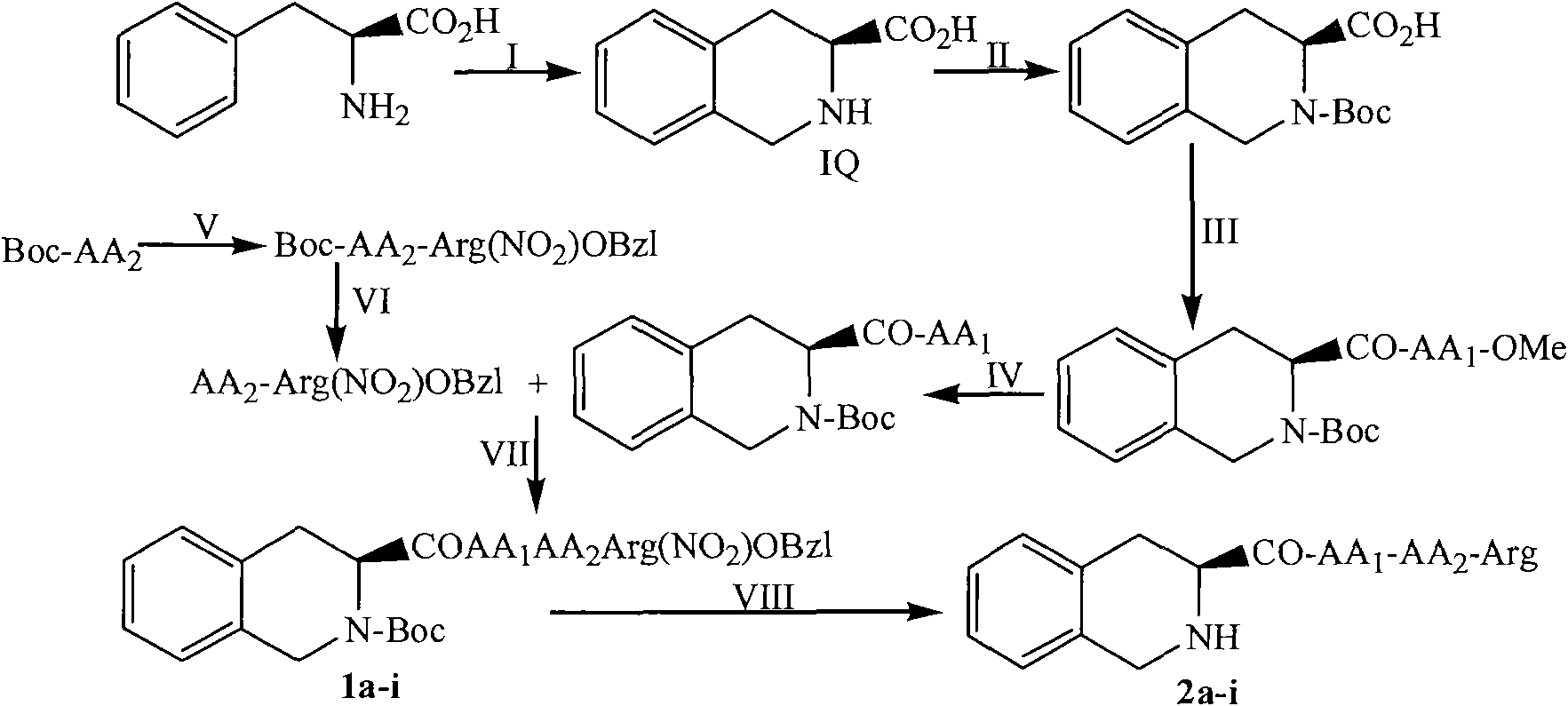
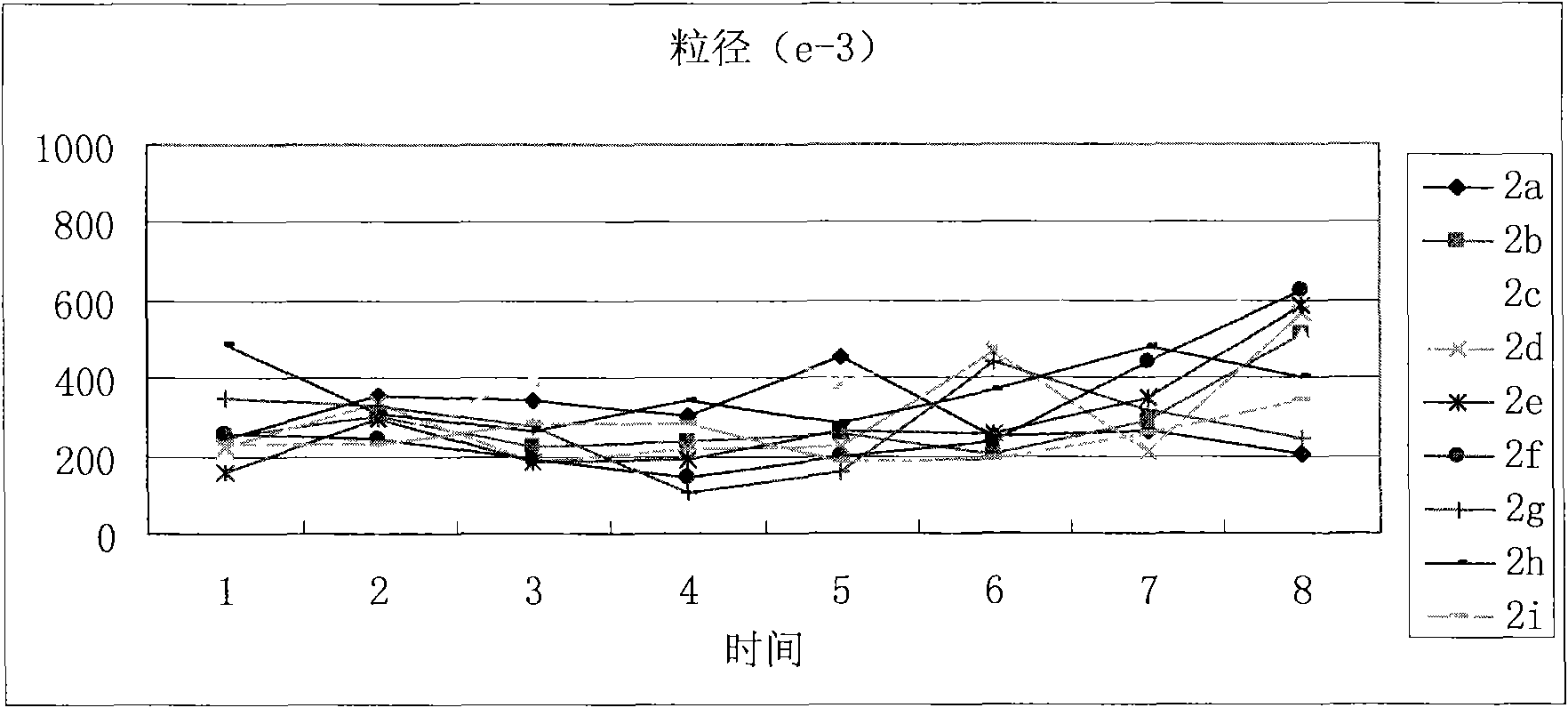
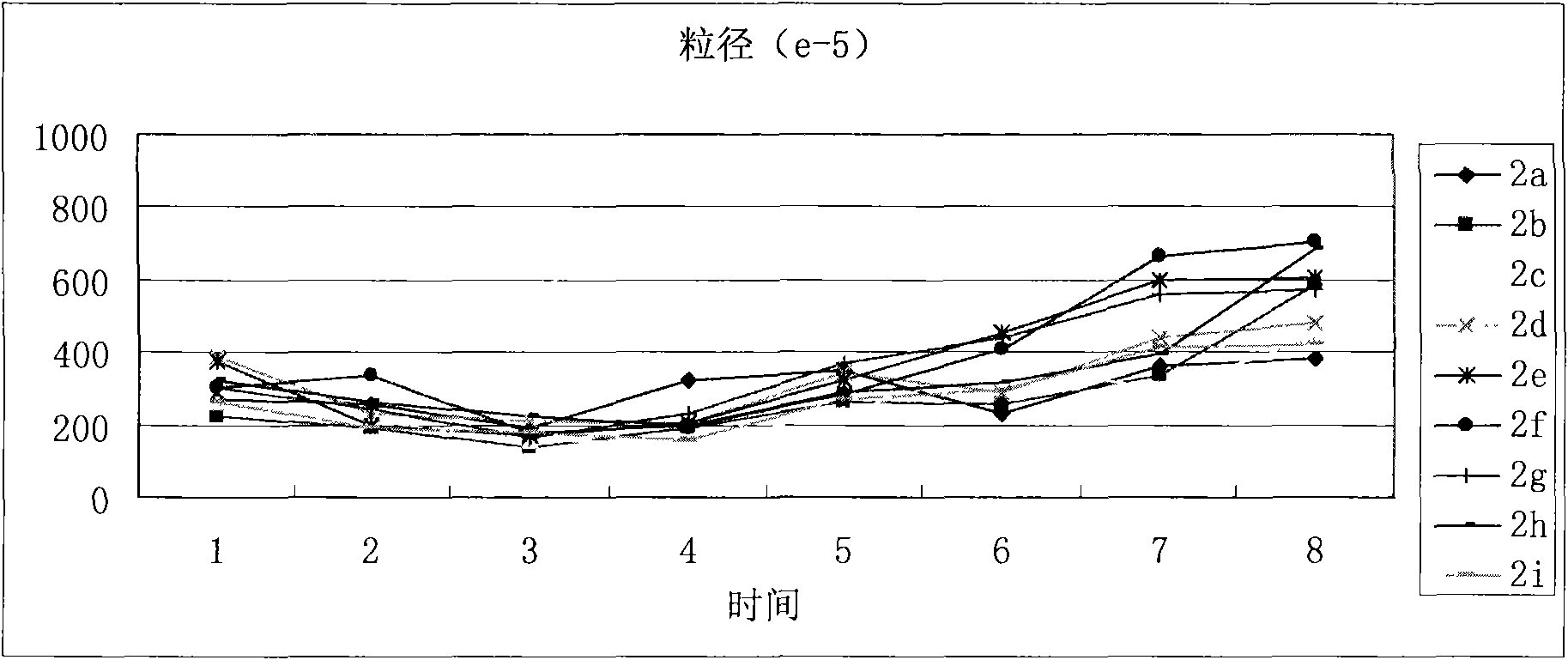
(3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid kyrine conjugate, preparation method and application thereof
A tetrahydroisoquinoline, -aa1-aa2-arg technology, applied in the field of conjugates and antithrombotic agents, can solve the problems of low water solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0027] To 4.0 g (24.2 mmol) of L-phenylalanine, 21.6 ml of formaldehyde was first added dropwise, and then 36 ml of 35% concentrated hydrochloric acid was added dropwise. The obtained suspension was heated in an oil bath to 90-100°C and stirred for 2 hours to completely dissolve the phenylalanine. After 2.5 hours of reaction, a white precipitate began to form. After 7 hours of reaction, TLC (CHCl 3 / CH 3OH=5 / 1) showed the disappearance of L-phenylalanine, suction filtered to obtain 4.2g light yellow solid, which was dried, and then the gained egg yellow solid was added to 86ml of ethanol (80%) and heated in an oil bath at 80°C to The colorless solid was dissolved, cooled to room temperature, and 2mol / ml NaOH potassium hydroxide solution was slowly added dropwise, and a colorless precipitate was precipitated. When the precipitate reached the maximum, 4.17 g (97.5%) of the title compound was...
Embodiment 2
[0028] Example 2 Preparation of (3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0029] Dissolve 2.49g (62.15mmol) sodium hydroxide into 62.2ml water under ice bath, then add 10g (56.49mmol) (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid to prepare suspension. Dissolve 14.8g (67.8mmol) (Boc) in 40ml tetrahydrofuran 2 O, added to the suspension. The reaction mixture was stirred for 24 hours, and the CO produced by the reaction was continuously removed during the reaction. 2 , when the solution became clear, TLC (methanol / chloroform: 1:10) showed that (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid disappeared, and the reaction was stopped. The reaction mixture was concentrated under reduced pressure to remove tetrahydrofuran, and the resulting oil was dissolved in ethyl acetate. The resulting solution was sequentially washed with 5% KHSO 4 Wash with saturated NaCl aqueous solution. The organic layer was dried over anhydrous sodium sulfate, filter...
Embodiment 3
[0030] Example 3 Preparation of N-[(3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline-3-formyl]-glycine methyl ester
[0031] Add 0.151g (1.109mmol ) N-hydroxybenzotriazole (HOBt), after stirring for 10 minutes, add 0.228g (1.08mmol) dicyclohexylcarbodiimide (DCC) to obtain the reaction solution (I). Suspend 0.136g (0.102mmol) of HCl·Gly-OMe in 4ml of anhydrous THF, add 1ml of N-methylmorpholine (NMM) to adjust the pH value to 8-9, and stir to obtain the reaction solution (II). Add (I) into (II), stir at room temperature for 12h, TLC (ethyl acetate / petroleum ether, 1:3) showed that (3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline- 3-Carboxylic acid disappears. Dicyclohexylurea (DCU) was filtered off, the filtrate was concentrated to dryness under reduced pressure, and the residue was dissolved in 50 ml of ethyl acetate. The resulting solution was sequentially washed with 5% NaHCO 3 Wash 3 times with aqueous solution, 3 times with saturated NaCl aqueous solution, 5% KHSO 4 Wash 3 times wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com