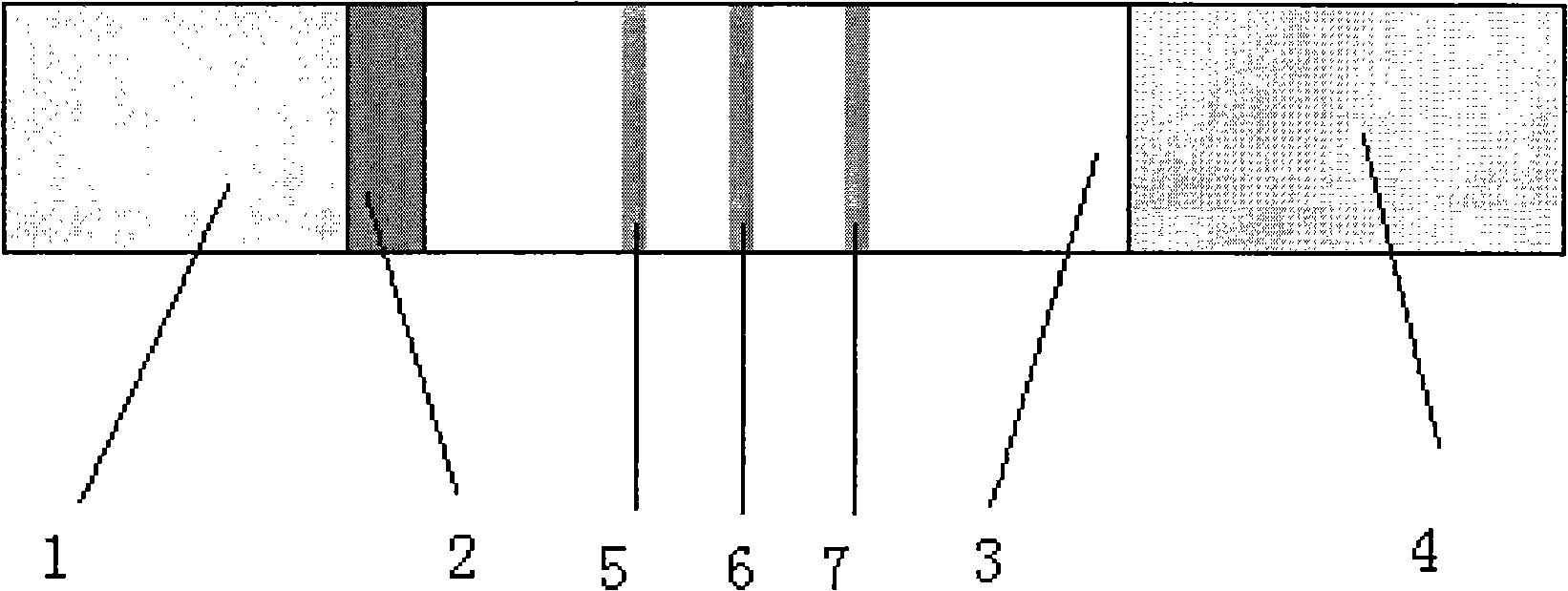
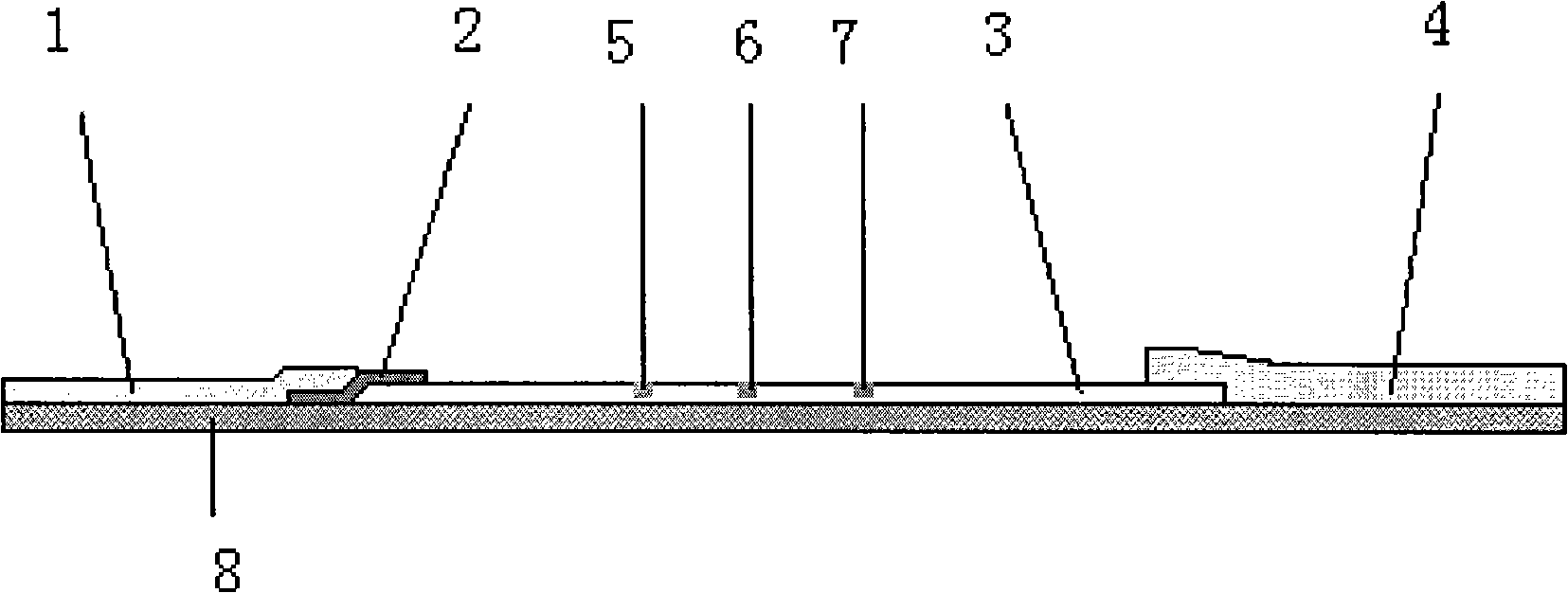
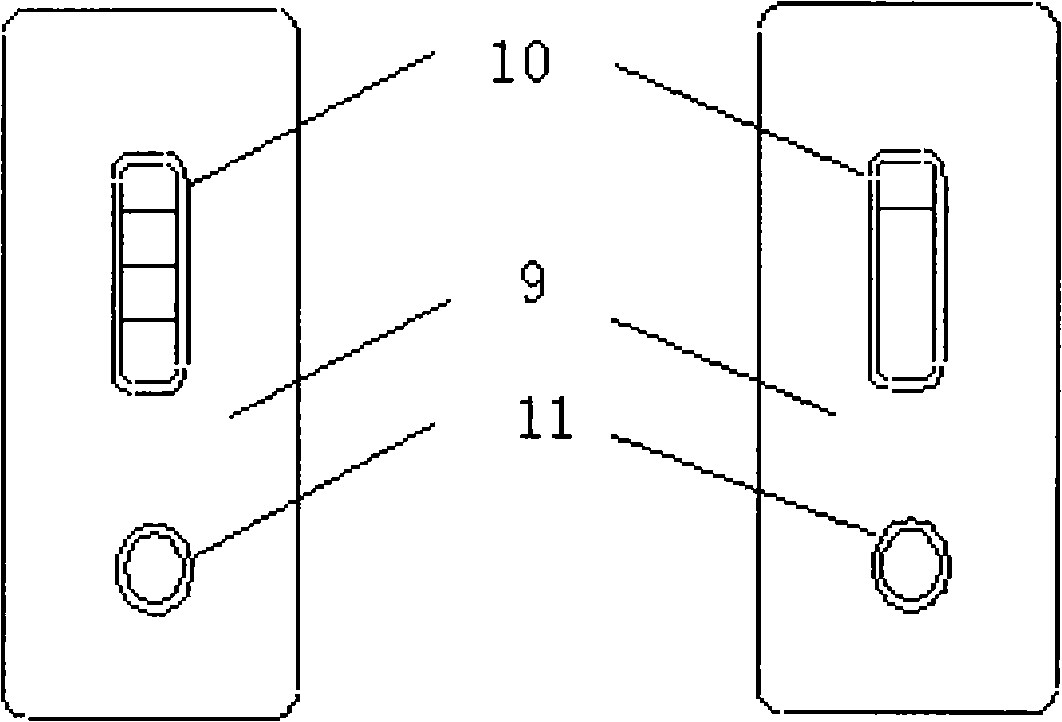
Reagent for detecting acute myocardial infarction by immunological method and test strip
A technology for acute myocardial infarction and test strips, applied in biochemical equipment and methods, biological testing, anti-animal/human immunoglobulin, etc., can solve problems such as the use of other indicators, long detection time, and complicated operations, and achieve The effect of improving sensitivity and specificity and improving diagnostic accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1.H-FABP, cTnI recombinant antigen
[0061] Preparation of H-FABP recombinant antigen:
[0062] According to the human H-FABP DNA sequence published by GENBANK, two PCR primers containing restriction endonuclease sites at both ends were designed and synthesized. The sequence is
[0063] 5'-AGCTGGATCCATGGTGGACGCTTTCCTGGGC-3';
[0064] 5'-CTAGGAATTCTGCCTCTTTCTCATAAGTGCG-3'.
[0065] The cDNA sequence of H-FABP was amplified from the heart tissue by RT-PCR, inserted into the expression vector pET16b, and after the gene sequence was confirmed to be correct by sequencing, it was transferred into E.coli BL / 21 (LysE) strain, induced by IPTG, and expressed The H-FABP was separated and purified by Ni-Agarose affinity chromatography column to obtain H-FABP recombinant antigen. The specific operation was carried out according to the method taught in "Molecular Cloning" and the product manual.
[0066] Preparation of cTnI recombinant antigen:
[0...
Embodiment 2
[0071] The preparation of embodiment 2.H-FABP, cTnI monoclonal antibody
[0072] Immunize each BALB / c mouse with intraperitoneal injection of 100 μg of the suspension of the above-mentioned purified recombinant protein + Freund’s complete adjuvant for the first time, and then inject 100 μg of the suspension of purified recombinant protein + Freund’s incomplete adjuvant every 1 month 1 time, a total of 3 times, and 3 days before the spleen was killed for cell fusion, the antigen was directly injected into the tail vein to boost the immunization once.
[0073] Production and screening of hybridoma cells The fusion of SP2 / 0 tumor cells and splenocytes of immune mice and the cloning of hybridoma cells were carried out according to the routine methods of our laboratory. Coat the ELISA plate with the above-mentioned purified recombinant protein antigen, use the indirect ELISA method to detect and screen the positive wells of the hybridoma cell culture supernatant, clone and culture ...
Embodiment 3
[0085] Example 3. Preparation of colloidal gold-labeled antibody solution
[0086] Prepare H-FABP, cTnI-specific antibody-labeled colloidal gold gold-labeled probe solution and gold-labeled antibody pad:
[0087] Colloidal gold is prepared by citric acid reduction method: 0.01% HAuCl4 (Shanghai Trial Brand purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd.) aqueous solution is boiled with a mass percentage concentration, and 2 mL of trisodium citrate with a mass percentage concentration of 1% is added under stirring Aqueous solution, continue to boil until the liquid is dark red to obtain a colloidal gold solution.
[0088] Determine the saturation of the colloidal gold-coupled antibody: adjust the pH value to 8.5 with 0.1M K2CO3 solution, prepare a 96-well microtiter plate, and dilute the specific antibody against H-FABP and cTnI with 0.05M boric acid buffer in the well , make a dilution gradient, add the same volume of colloidal gold and mix well, then add the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com