High-sensitivity magnetic particle chemiluminescence immunoassay kit for cardiac troponin I (hs-cTnI) and preparation method and purpose thereof
A technology of cardiac troponin and magnetic particles, which is applied in the field of medical testing, can solve problems such as the inability to detect changes in cTnI concentration, and achieve the effects of stable test results, wide detection range, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
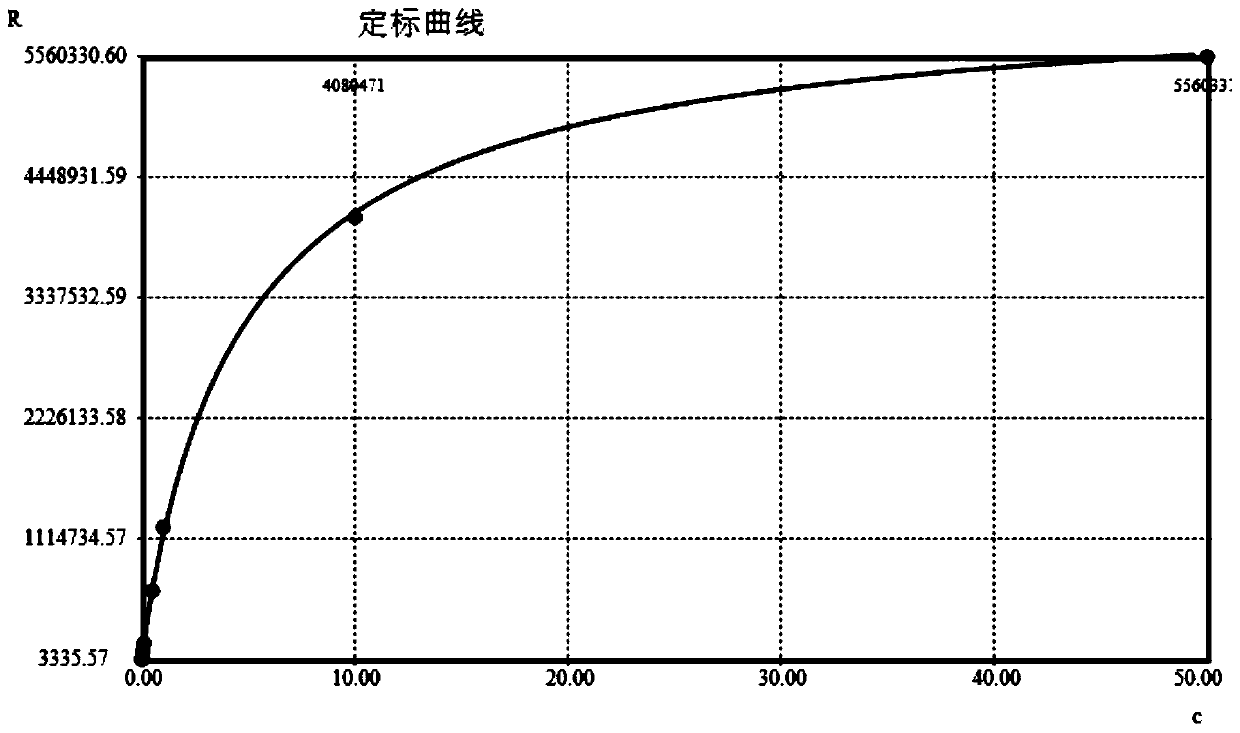
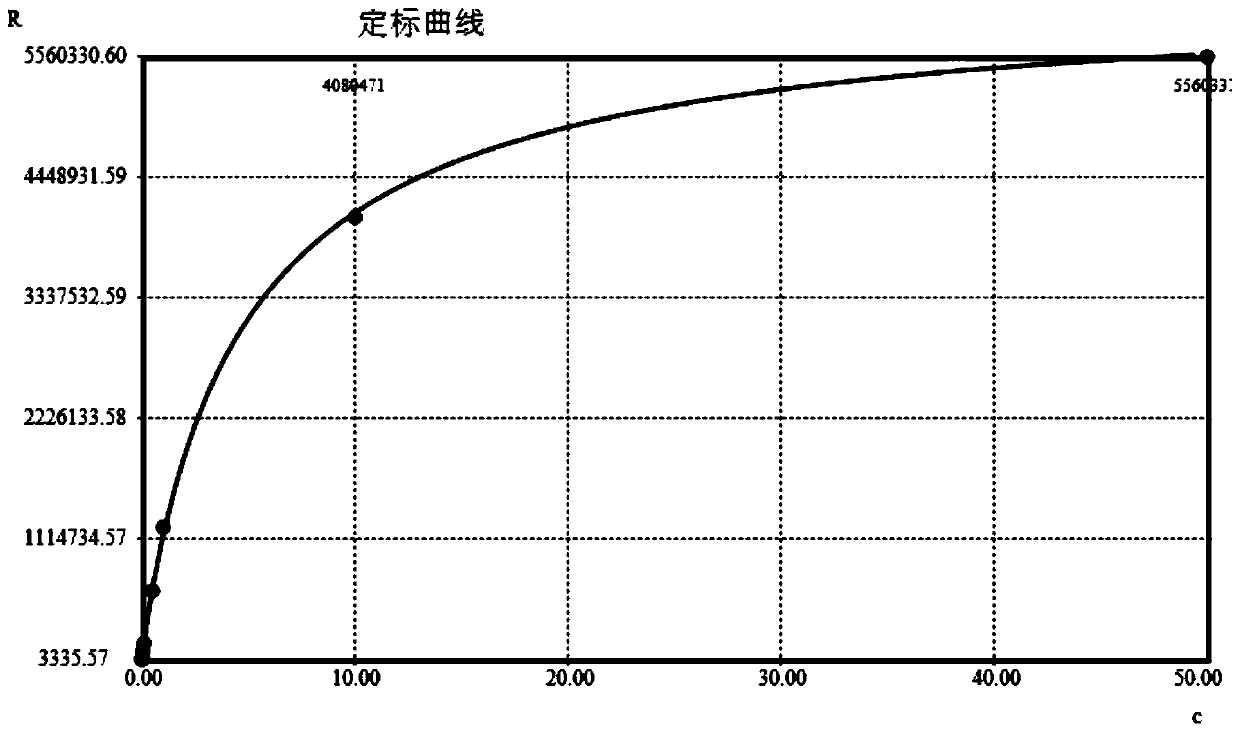
[0034] Example 1——Preparation of high-sensitivity cardiac troponin I (hs-cTnI) magnetic particle chemiluminescence immunoassay kit
[0035] 1. Preparation of Reagent R1
[0036] A. Preparation of biotin-labeled anti-cTnI polyclonal antibody: Dilute anti-cTnI polyclonal antibody (HyTest) with buffer to a concentration of 2 mg / mL, mix with biotin and react at room temperature for 1-3 hours for biotin labeling . The mass ratio of anti-cTnI polyclonal antibody to biotin is 5:1-20:1, wherein the biotin is excessive, so as to fully label the anti-cTnI polyclonal antibody. The composition of the buffer is 5-50mM Na 2 CO 3 +10-100mM NaHCO 3 , pH 8.0-10.5. After labeling, use a Thermo Scientific Zeba TM Spin Desalting Columns were subjected to centrifugal desalting and purification to obtain biotin-labeled anti-cTnI polyclonal antibody, which was stored at 4°C for future use.
[0037] B. Prepare the prepared biotin-labeled anti-cTnI polyclonal antibody with capture antibody dil...
Embodiment 2
[0052] Example 2——Preparation of high-sensitivity cardiac troponin I (hs-cTnI) magnetic particle chemiluminescence immunoassay kit
[0053] The preparation method of the present embodiment is similar to Example 1, but has the following differences:
[0054] 1. Preparation of Reagent R1
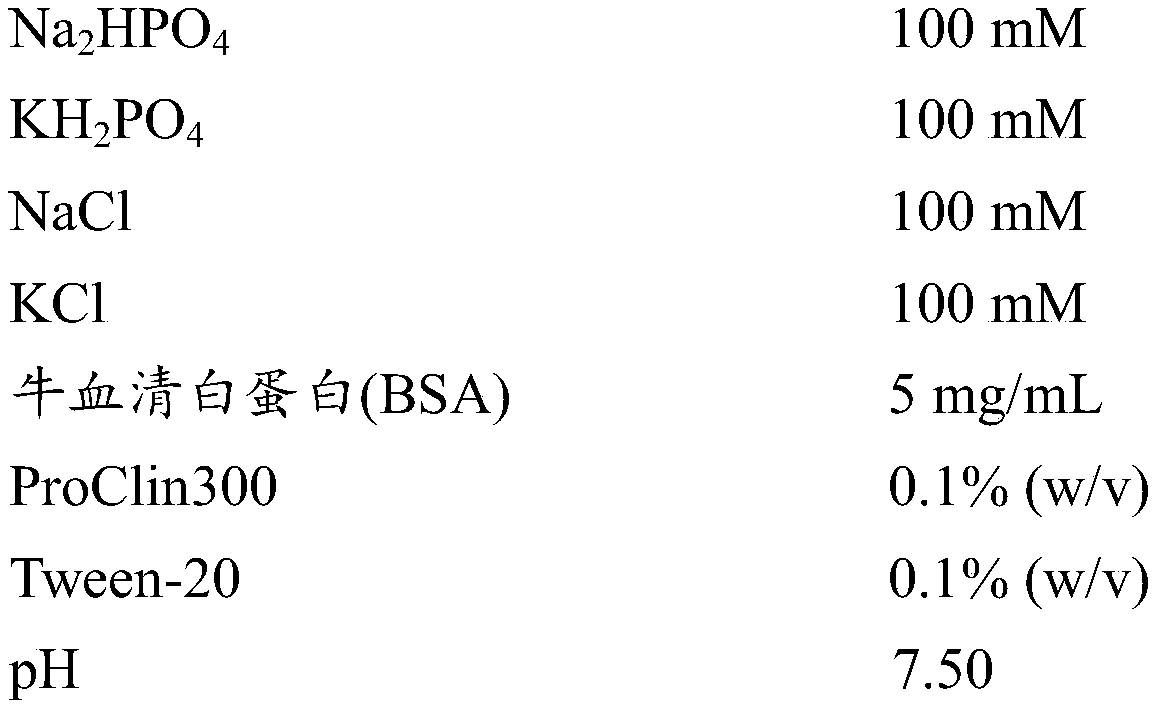
[0055] The prepared biotin-labeled anti-cTnI polyclonal antibody was adjusted to a concentration of 0.4 μg / mL with capture antibody diluent. The composition of the capture antibody diluent is shown in Table 4 below.
[0056] The composition of the capture antibody diluent of table 4 embodiment 2
[0057]
[0058] 2. Preparation of Reagent R2
[0059] The prepared two strains of acridinium ester-labeled anti-cTnI monoclonal antibodies targeting different cTnI epitopes were mixed at a mass ratio of 1:1, and then prepared to a concentration of 0.5 μg / mL with labeled antibody diluent. The composition of the labeled antibody diluent is shown in Table 5 below.
[0060] The composition of the...
Embodiment 3
[0067] Example 3——Preparation of high-sensitivity cardiac troponin I (hs-cTnI) magnetic particle chemiluminescence immunoassay kit
[0068] The preparation method of the present embodiment is similar to Example 1, but has the following differences:
[0069] 1. Preparation of Reagent R1
[0070] The prepared biotin-labeled anti-cTnI polyclonal antibody was adjusted to a concentration of 2 μg / mL with capture antibody diluent. The composition of the capture antibody diluent is shown in Table 7 below.
[0071] The composition of the capture antibody diluent of table 7 embodiment 3
[0072]
[0073] 2. Preparation of Reagent R2
[0074] The prepared two strains of acridinium ester-labeled anti-cTnI monoclonal antibodies targeting different cTnI epitopes were mixed at a mass ratio of 1:1, and then prepared at a concentration of 5 μg / mL with labeled antibody diluent. The composition of the labeled antibody diluent is shown in Table 8 below.
[0075] The composition of the lab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Analytical sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com