CTnI, Myo and CK-MB three-in-one colloidal gold test strip, kit thereof, and making methods of test strip and kit
A technology of CK-MB and detection test paper, which is applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of doctors' misjudgment or missed judgment, large detection error, etc., and achieve simple detection, easy popularization, and simplified operation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
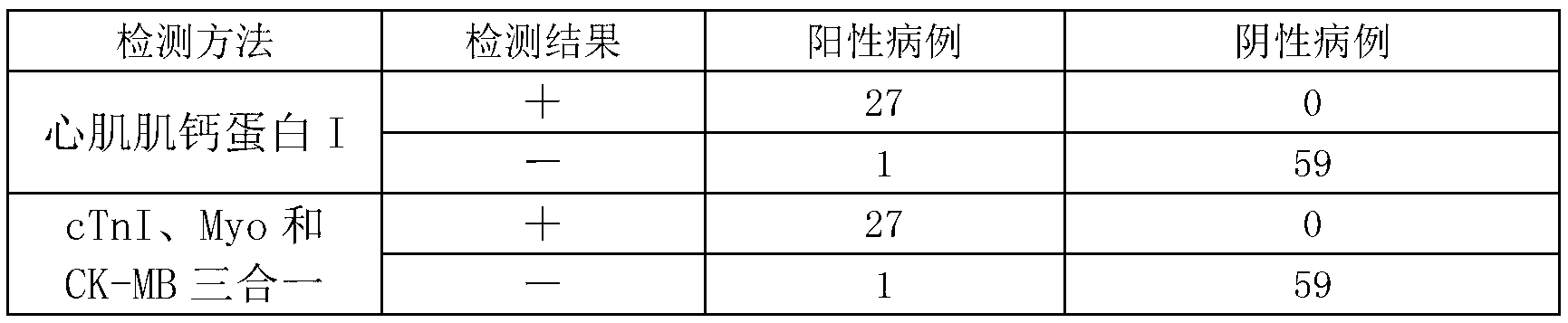
[0029] Example 1: Composition of Troponin I (cTnI), Myoglobin (Myo), Creatine Kinase Isoenzyme (CK-MB) three-in-one rapid detection test strip (colloidal gold method)
[0030] 1. Sample processing bottle: The sample processing bottle (purchased from Hangzhou Beiyu) is filled with 1mL sample processing solution (phosphate buffer solution, the main raw material is purchased from Sinopharm Group).
[0031] 2. Test strip: 60*5mm bottom plate (purchased from Shanghai Jieyi Company); 15*5mm thick fiber filter paper for absorbent pad (purchased from Shanghai Jieyi Company); 25*5mm nitrocellulose membrane (purchased from Millipore Company ) were sequentially coated with cardiac troponin I antibody (purchased from abcam company), myoglobin antibody (purchased from abcam company), creatine kinase isoenzyme antibody (purchased from abcam company), goat anti-mouse IgG antibody (purchased from abcam company) abcam Company); the sample pad (purchased from Shanghai Jieyi Company) was attache...
Embodiment 2
[0032] Embodiment 2: the preparation of kit
[0033] 1. Preparation of sample processing solution: Prepare sample processing solution: prepare phosphate buffer solution (pH7.0±0.2), dispense it into sample processing bottles, 1mL / bottle.
[0034]2. Preparation of test strips:
[0035] 2.1. Dilute human cTnI monoclonal antibody, human Myo monoclonal antibody, human CK-MB monoclonal antibody, and goat anti-mouse IgG antibody to a coating concentration of 1.0-1.2 mg / mL, and divide the four coating solutions into nitric acid respectively. At the designated position on the cellulose membrane, dry the coated nitrocellulose membrane and store it under the drying conditions of 20-30°C and relative humidity of 30-35% for 5 hours.
[0036] 2.2. Preparation of gold label pad: Colloidal gold was prepared by trisodium citrate reduction method, and a certain proportion of monoclonal antibody cTnI / Myo / CK-MB was slowly added to the gold sol, so that colloidal gold and antibody formed monoclo...
Embodiment 3
[0041] Embodiment 3 detection method
[0042] 3.1. Specimen collection
[0043] 1. Collection of whole blood specimens: Fingertip, earlobe end or vein specimens should be used as soon as possible after collection, and anticoagulant blood should be used within 24 hours to avoid blood fusion.
[0044] 2. Collection of serum / plasma specimens: Serum / plasma specimens are obtained by centrifugation after venous blood collection. Specimens can be stored at 2-8°C for one week. Long-term storage needs to be frozen, but repeated freezing and thawing should be avoided.
[0045] 3. Take 100-200ul of whole blood, serum, and plasma samples and drop them into the sample loading window. If the whole blood is relatively viscous, add 1-2 drops of unused sample processing solution to the sample loading window.
[0046] 4. The detection temperature is 15-30°C and the humidity is 40-60% is the best.
[0047] 5. Interpret the result after 10-15 minutes:
[0048] 5.1. If the sample contains myogl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com