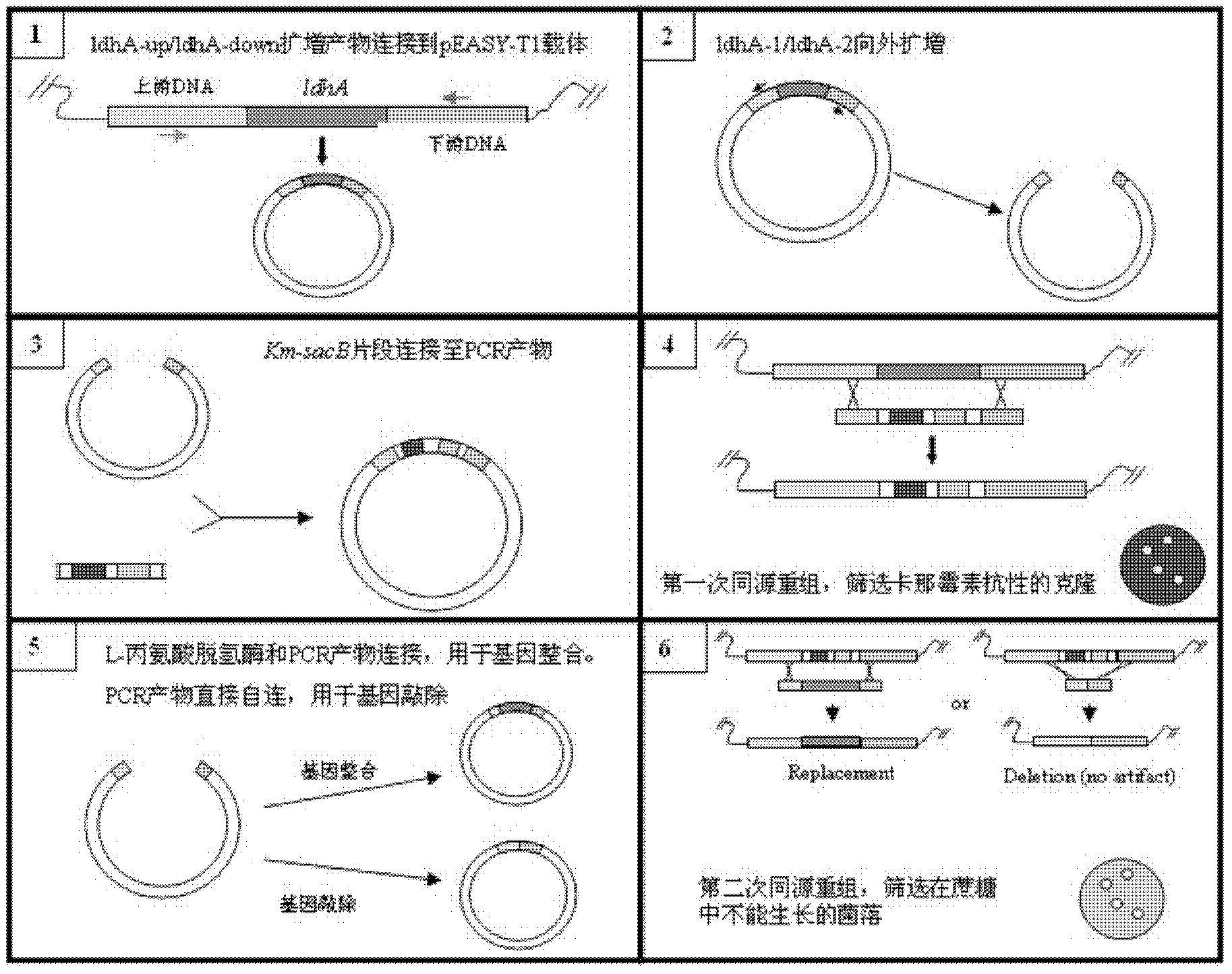
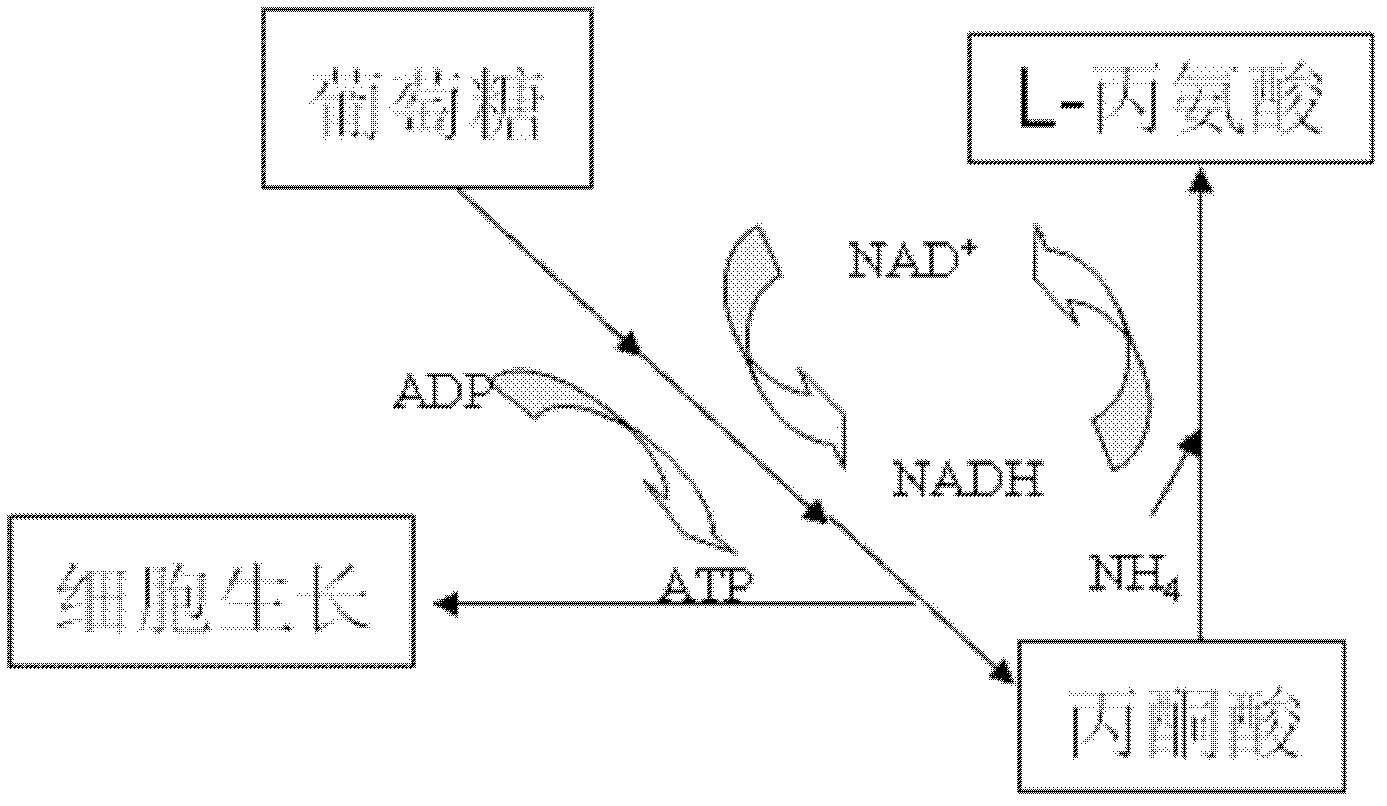
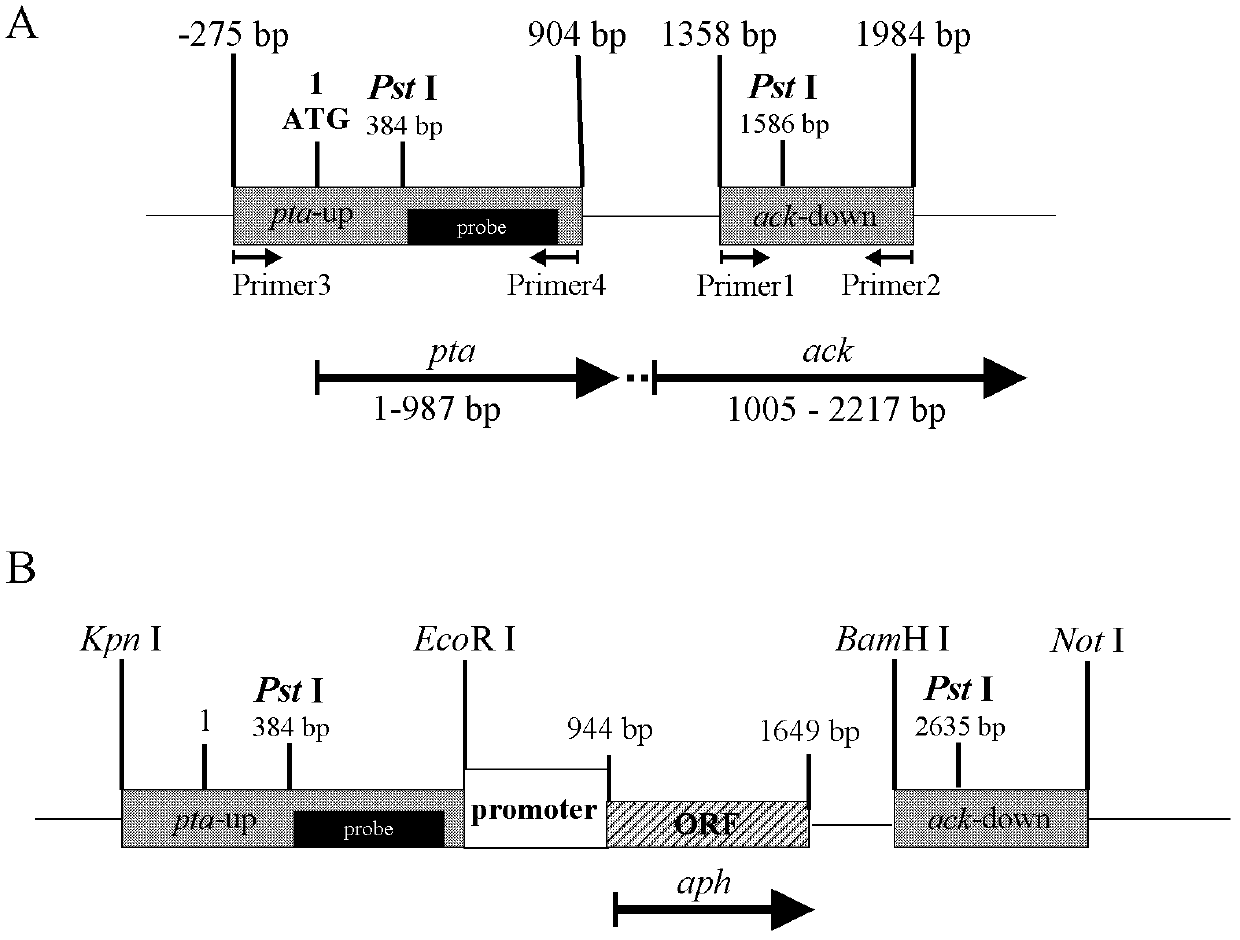
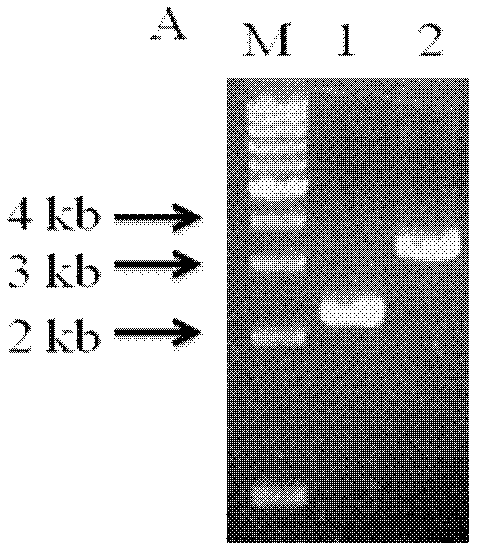
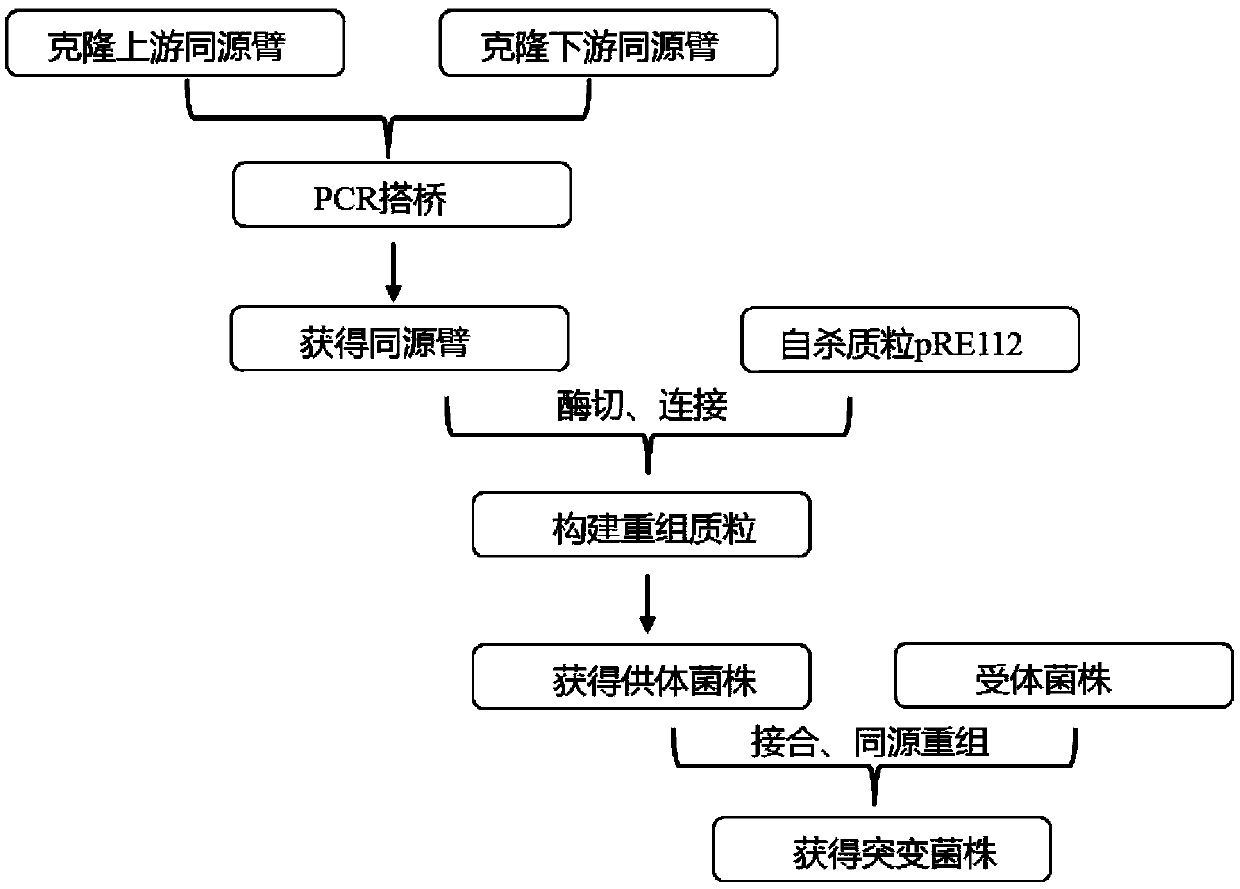
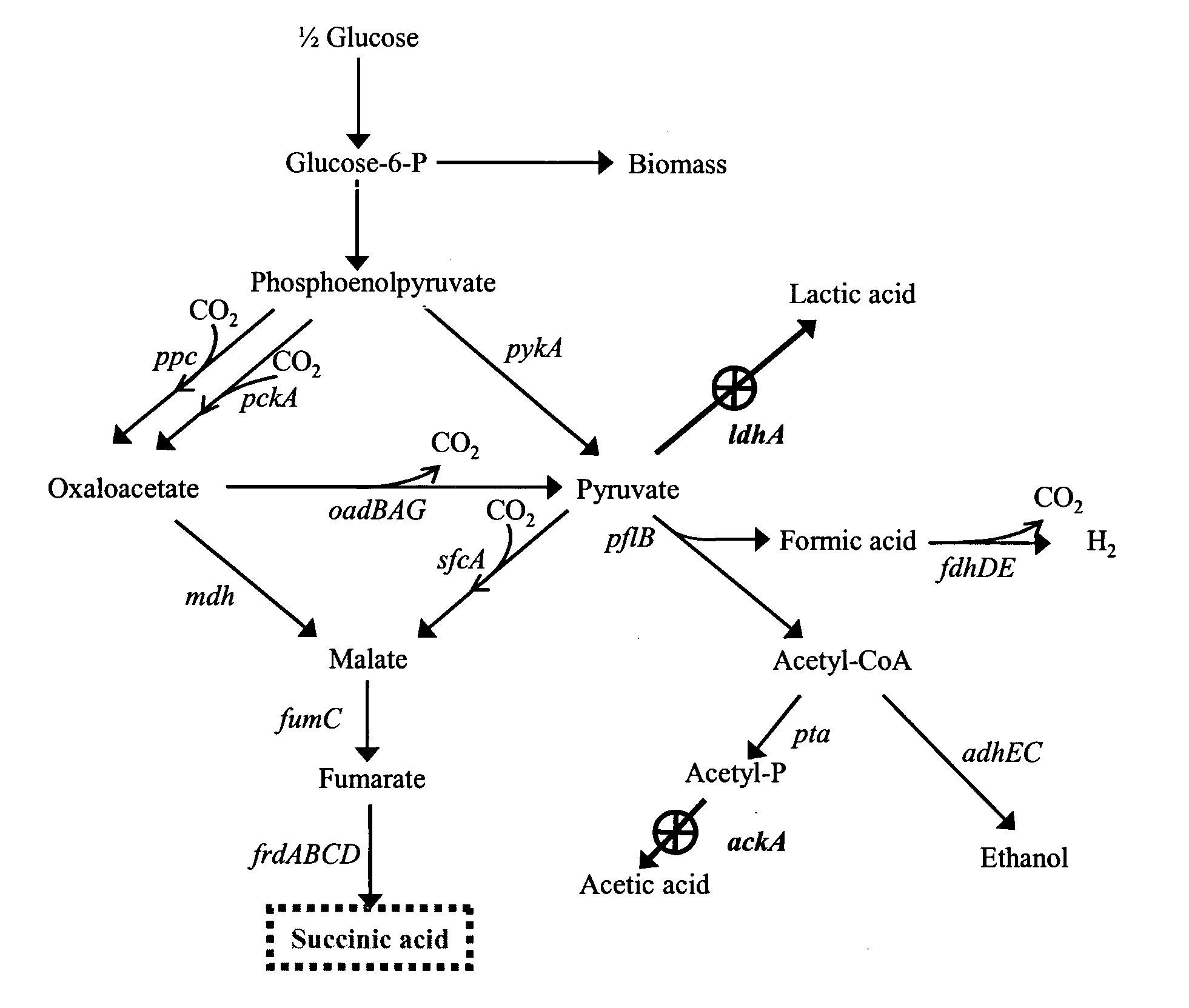
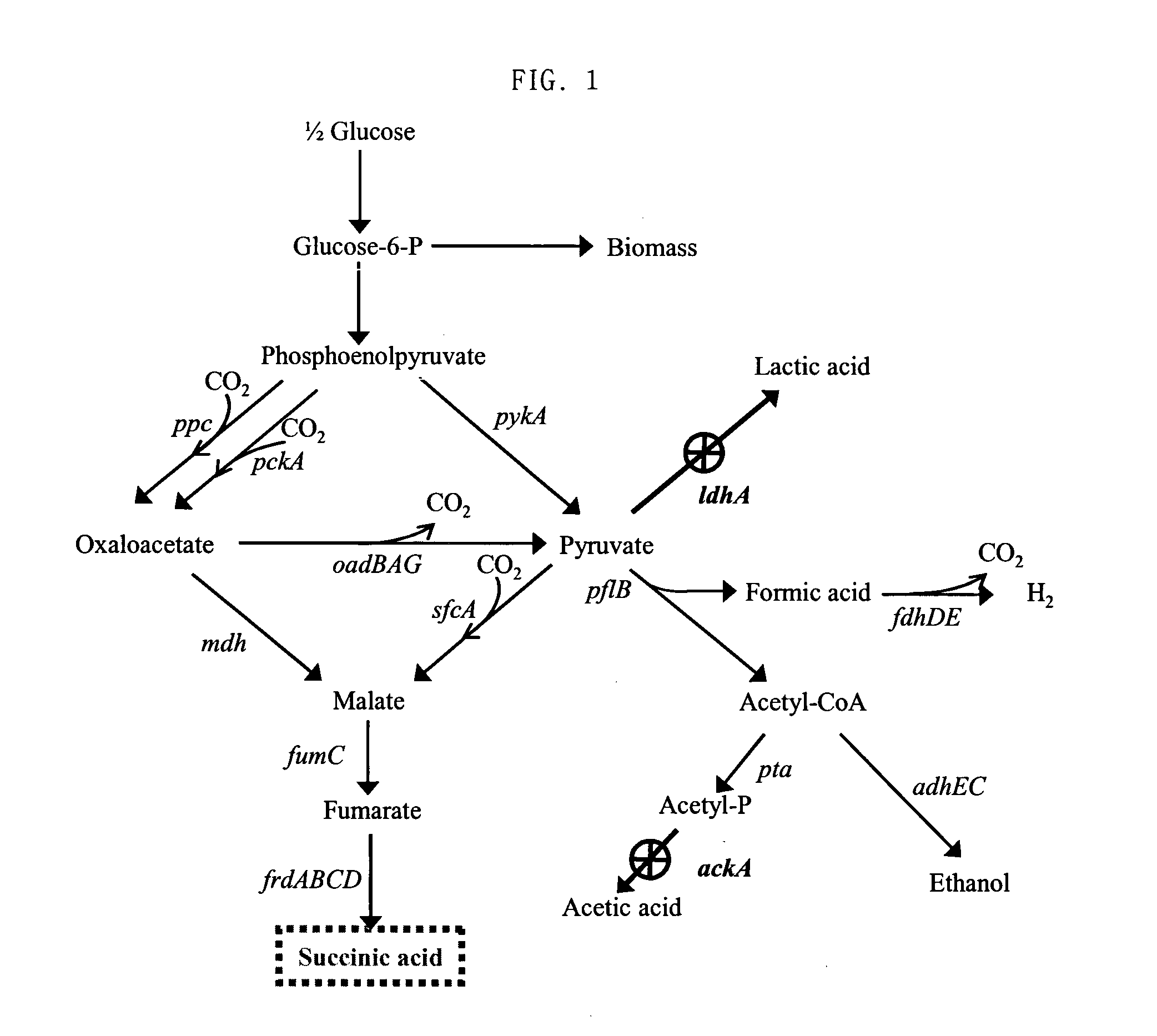
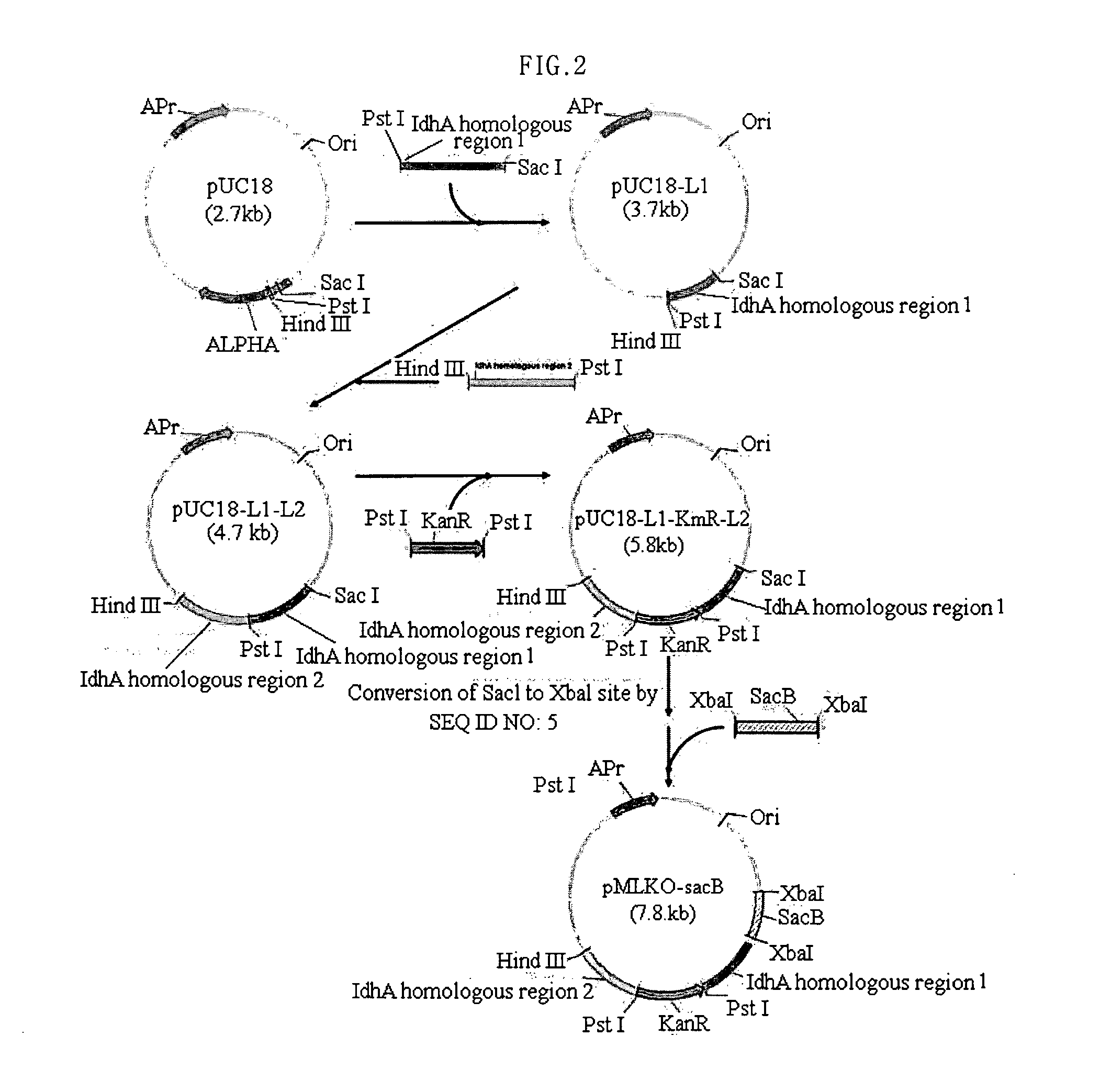
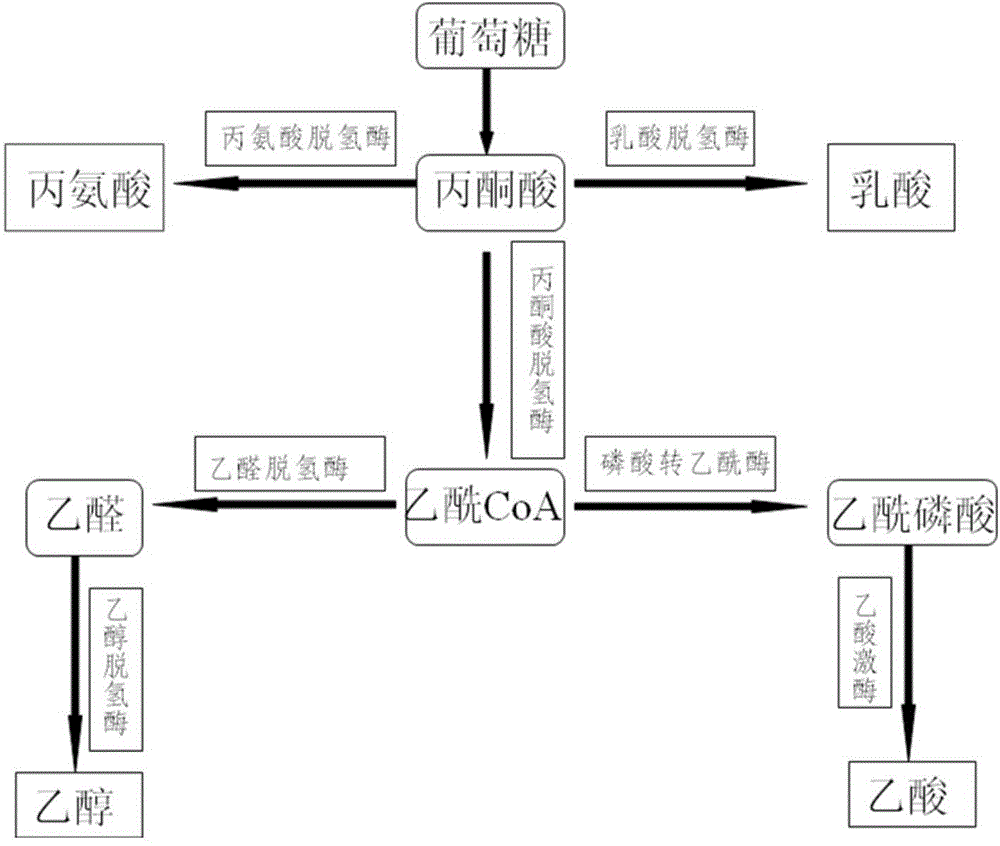
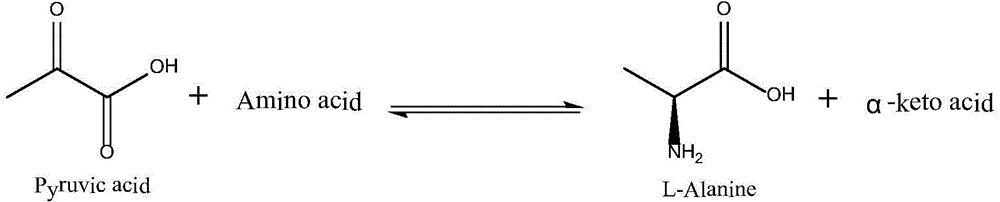
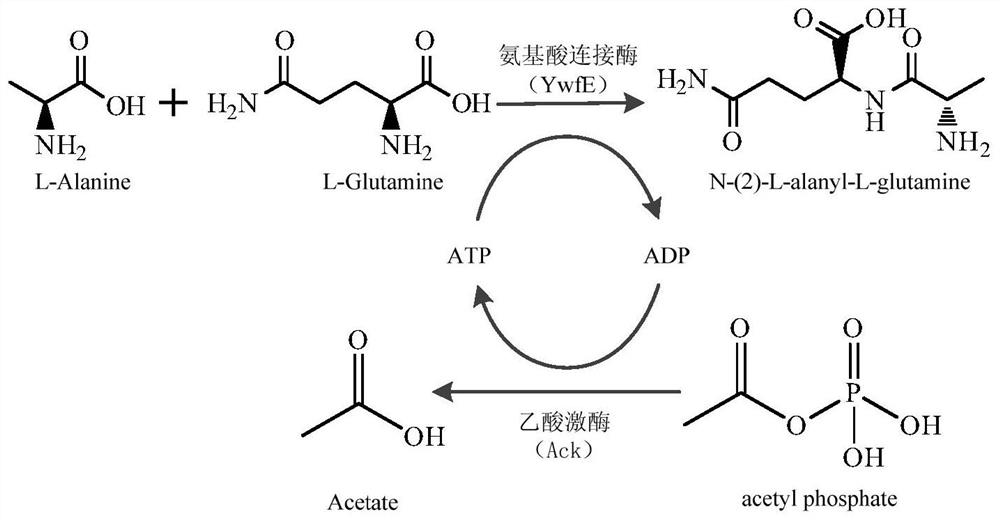
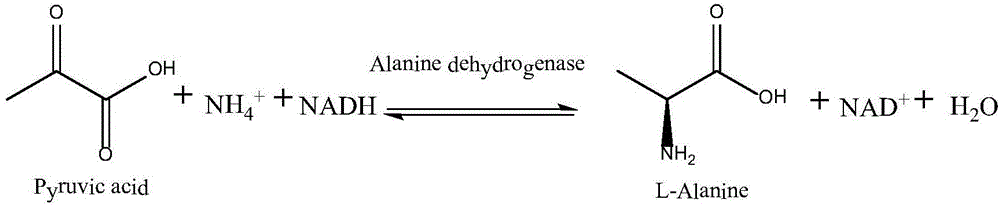Patents
Literature
59 results about "Acetate kinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
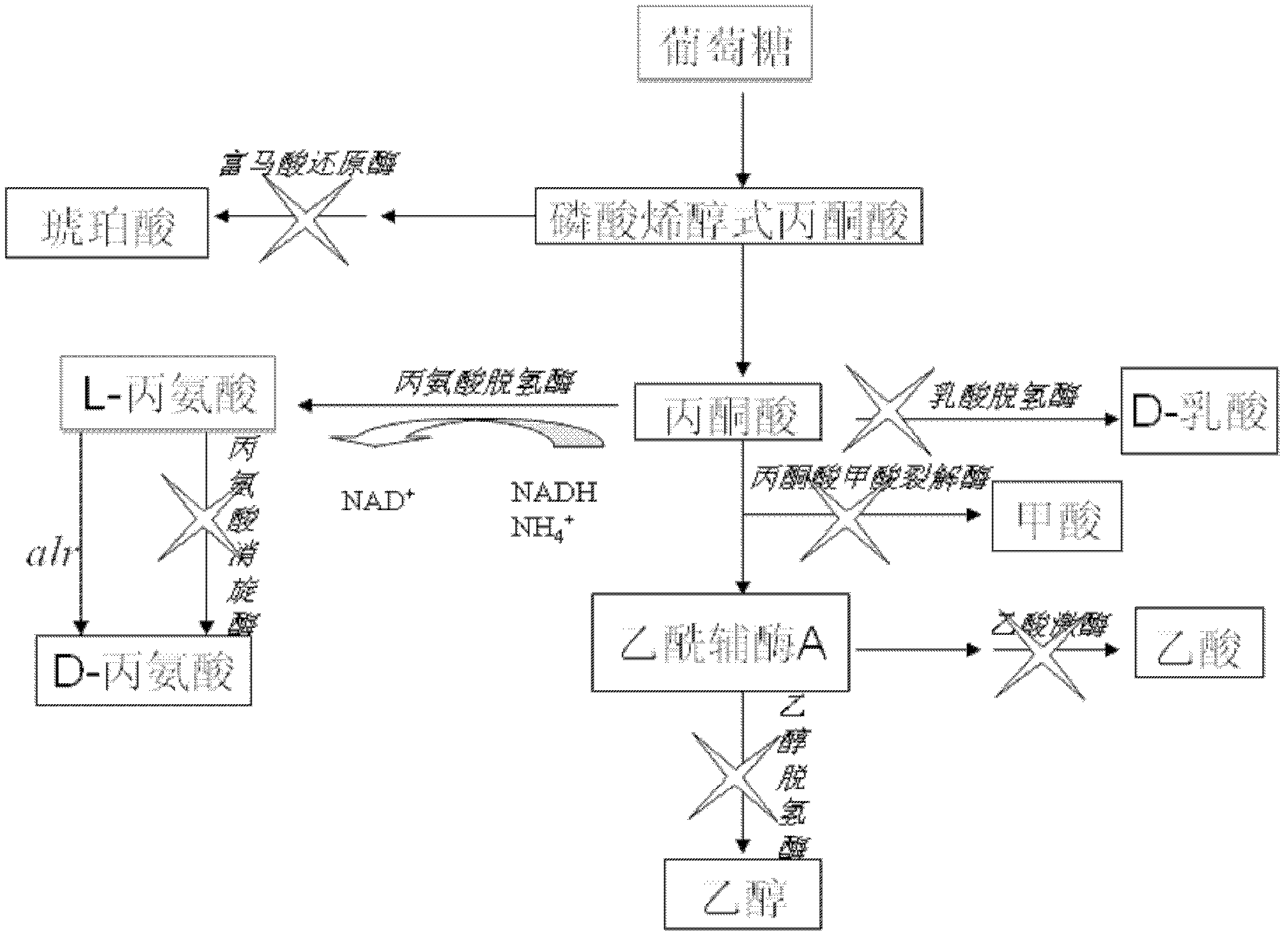
In molecular biology, acetate kinase (EC 2.7.2.1), which is predominantly found in micro-organisms, facilitates the production of acetyl-CoA by phosphorylating acetate in the presence of ATP and a divalent cation. Short-chain fatty acids (SCFAs) play a major role in carbon cycle and can be utilized as a source of carbon and energy by bacteria. Salmonella typhimurium propionate kinase (StTdcD) catalyzes reversible transfer of the γ-phosphate of ATP to propionate during l-threonine degradation to propionate. Kinetic analysis revealed that StTdcD possesses broad ligand specificity and could be activated by various SCFAs (propionate>acetate≈butyrate), nucleotides (ATP≈GTP>CTP≈TTP; dATP>dGTP>dCTP) and metal ions (Mg²⁺≈Mn²⁺>Co²⁺). Inhibition of StTdcD by tricarboxylic acid (TCA) cycle intermediates such as citrate, succinate, α-ketoglutarate and malate suggests that the enzyme could be under plausible feedback regulation. Crystal structures of StTdcD bound to PO₄ (phosphate), AMP, ATP, Ap4 (adenosine tetraphosphate), GMP, GDP, GTP, CMP and CTP revealed that binding of nucleotide mainly involves hydrophobic interactions with the base moiety and could account for the broad biochemical specificity observed between the enzyme and nucleotides. Modelling and site-directed mutagenesis studies suggest Ala88 to be an important residue involved in determining the rate of catalysis with SCFA substrates. Molecular dynamics simulations on monomeric and dimeric forms of StTdcD revealed plausible open and closed states, and also suggested role for dimerization in stabilizing segment 235-290 involved in interfacial interactions and ligand binding. Observation of an ethylene glycol molecule bound sufficiently close to the γ-phosphate in StTdcD complexes with triphosphate nucleotides supports direct in-line phosphoryl transfer. The enzyme is important in the process of glycolysis, enzyme levels being increased in the presence of excess glucose. The growth of a bacterial mutant lacking acetate kinase has been shown to be inhibited by glucose, suggesting that the enzyme is involved in excretion of excess carbohydrate. A related enzyme, butyrate kinase, facilitates the formation of butyryl-CoA by phosphorylating butyrate in the presence of ATP to form butyryl phosphate.
Thermophilic Organisms For Conversion Of Lignocellulosic Biomass To Ethanol
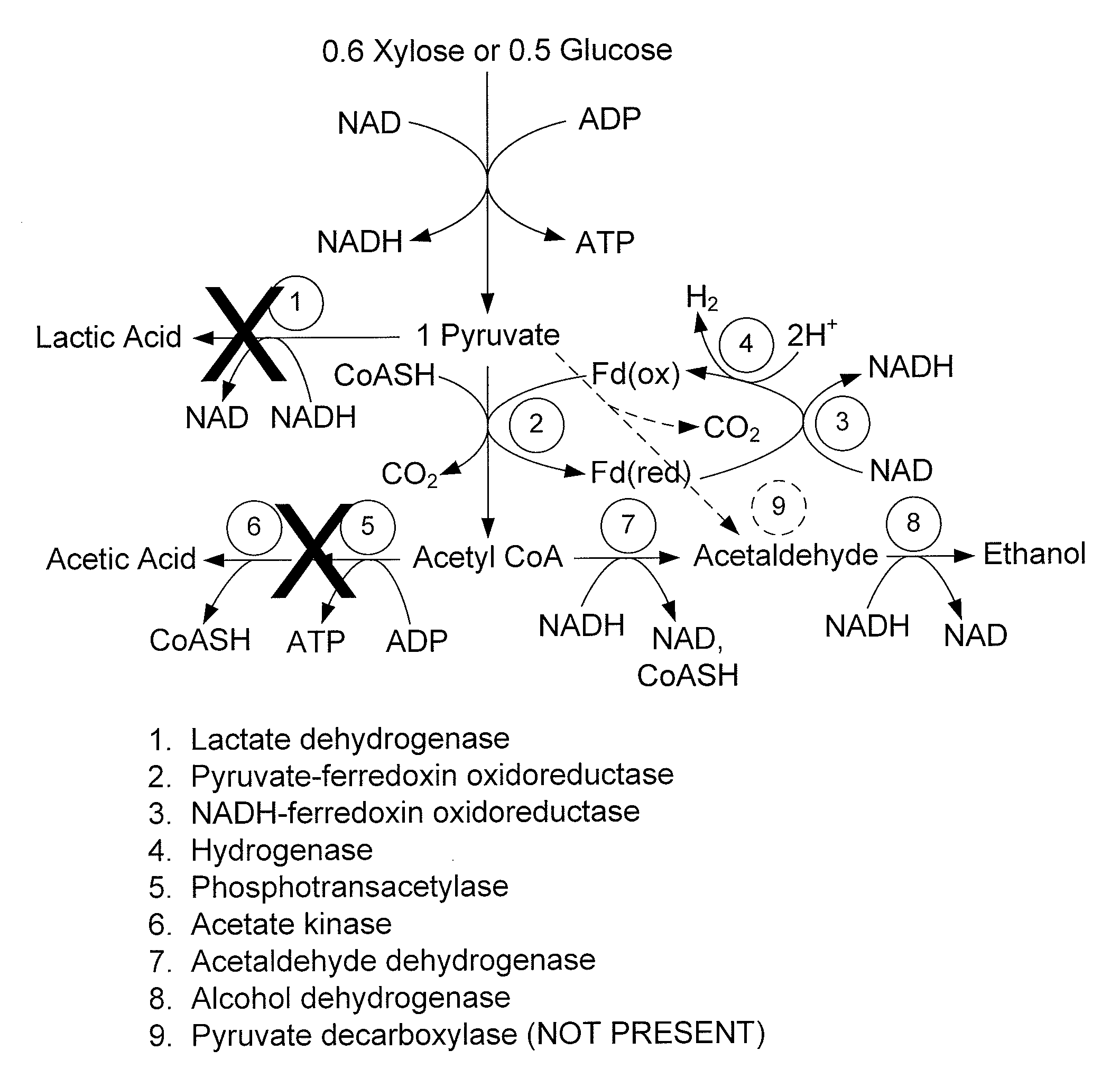
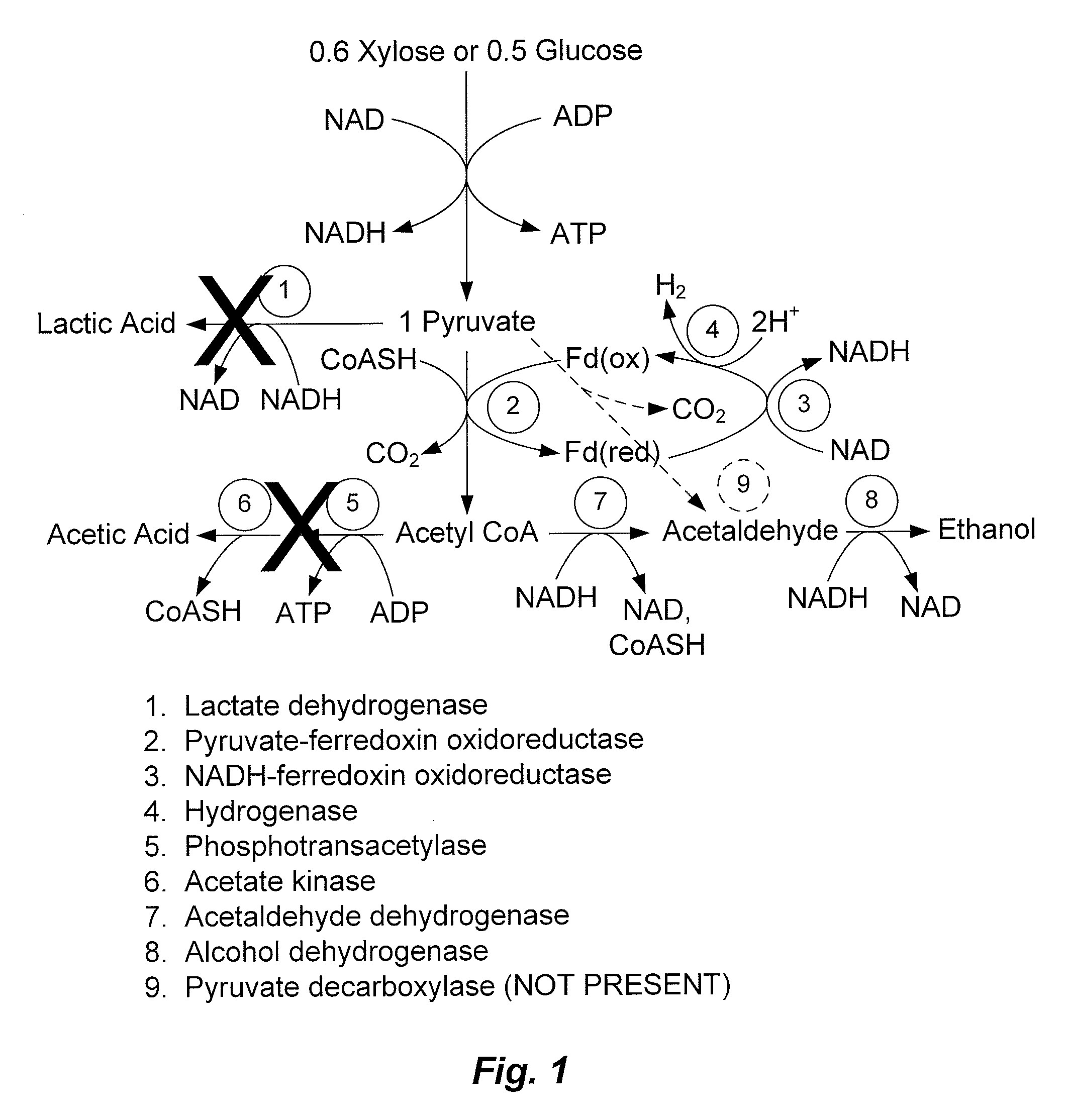
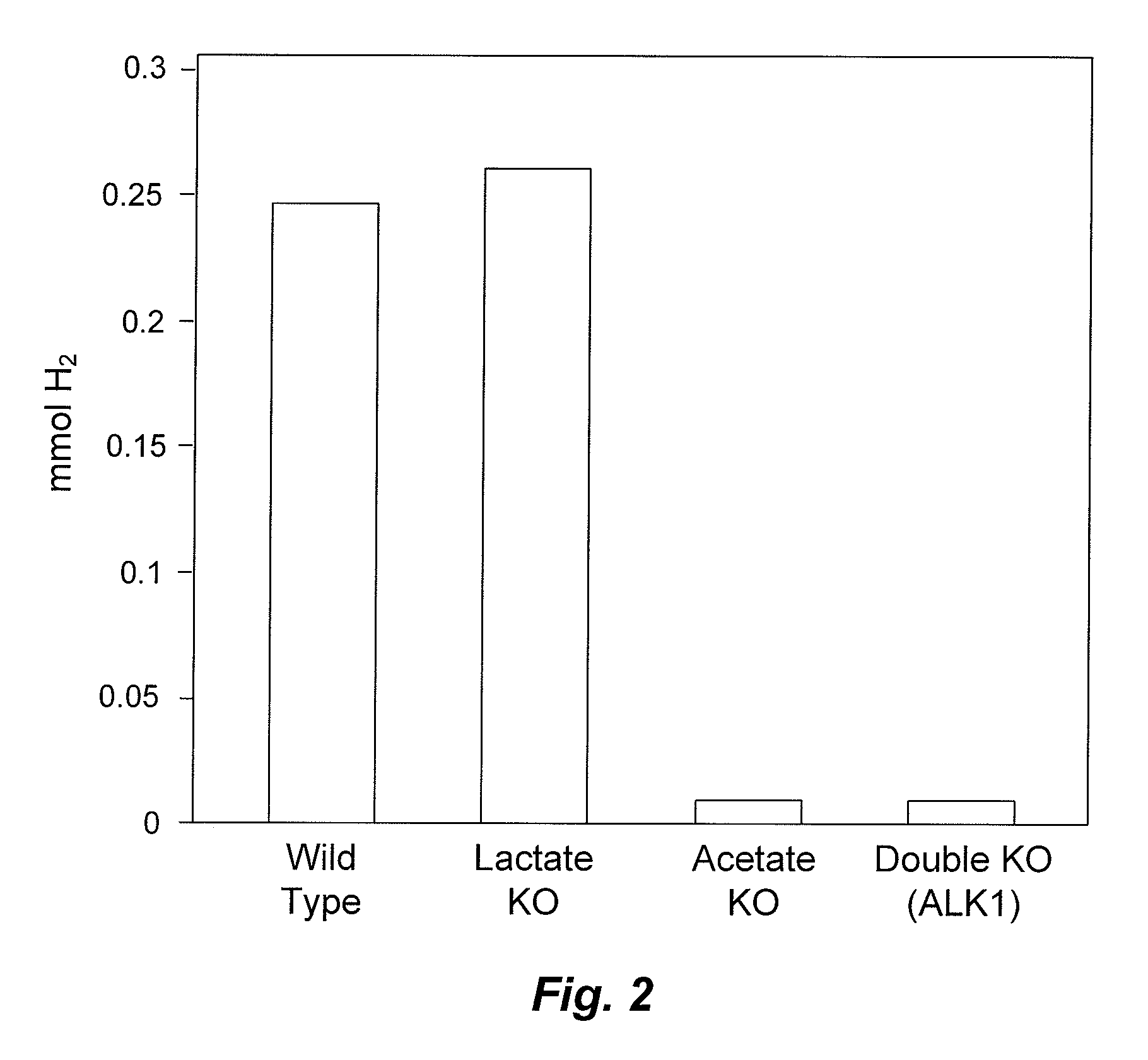
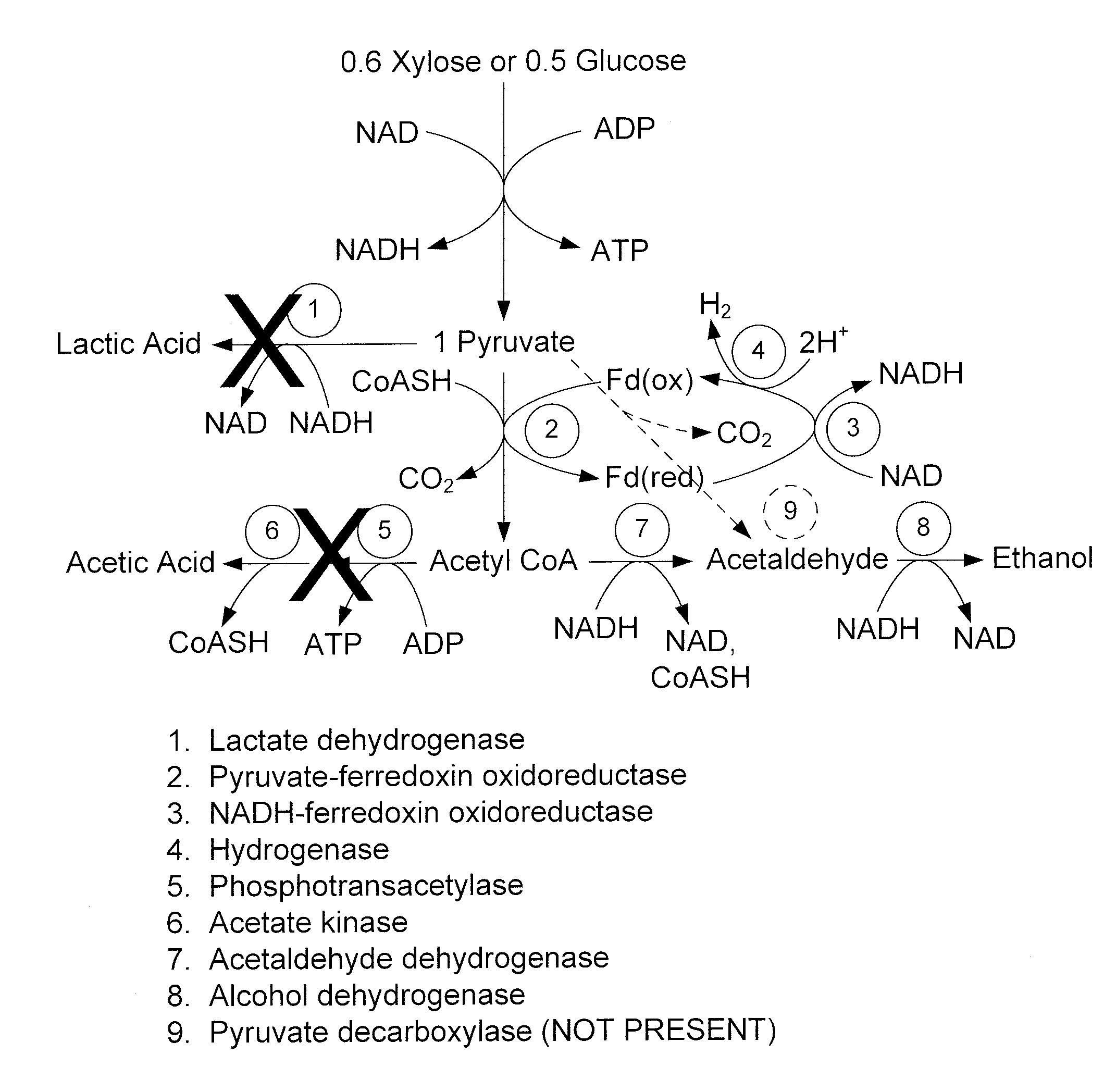
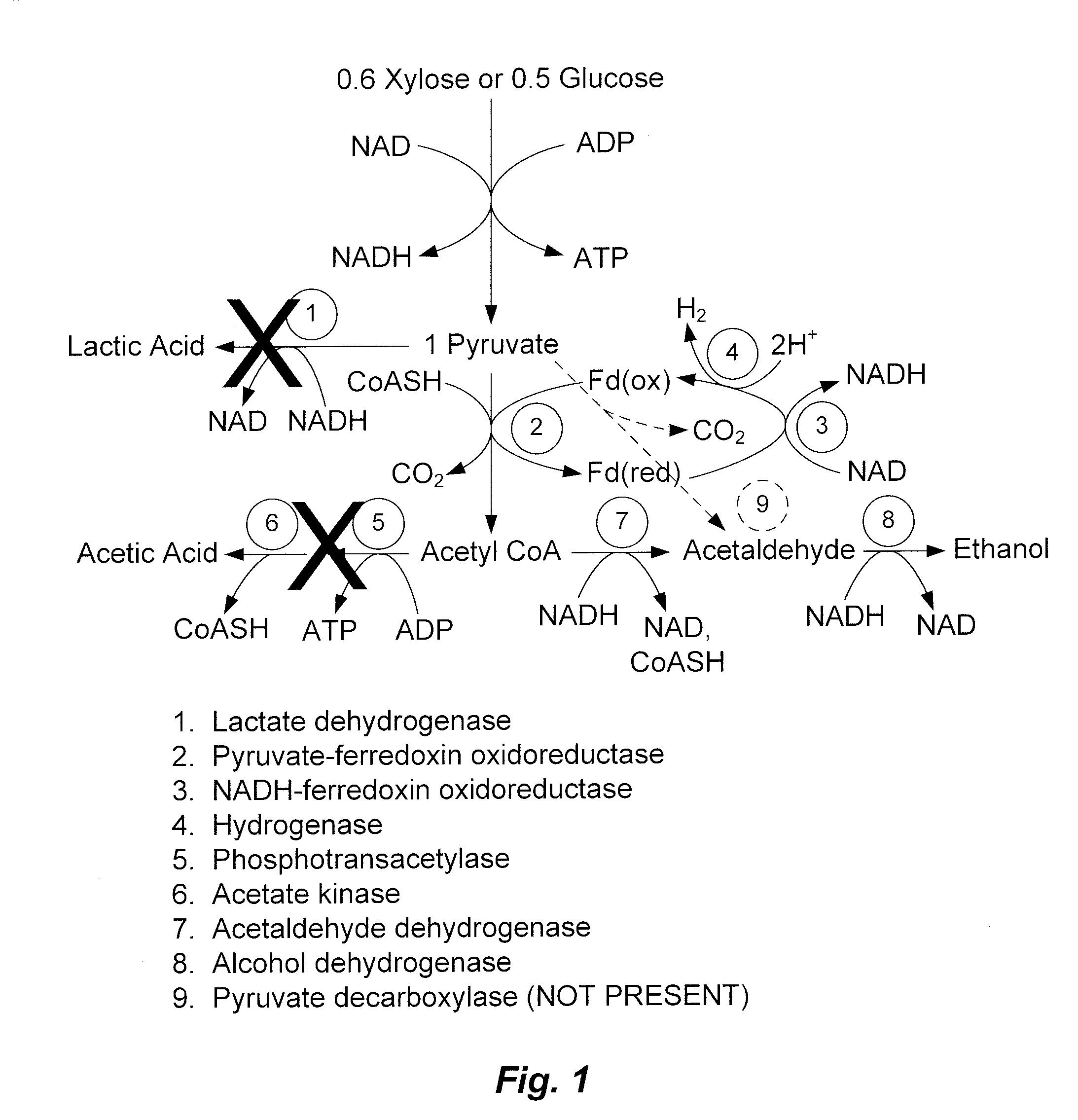
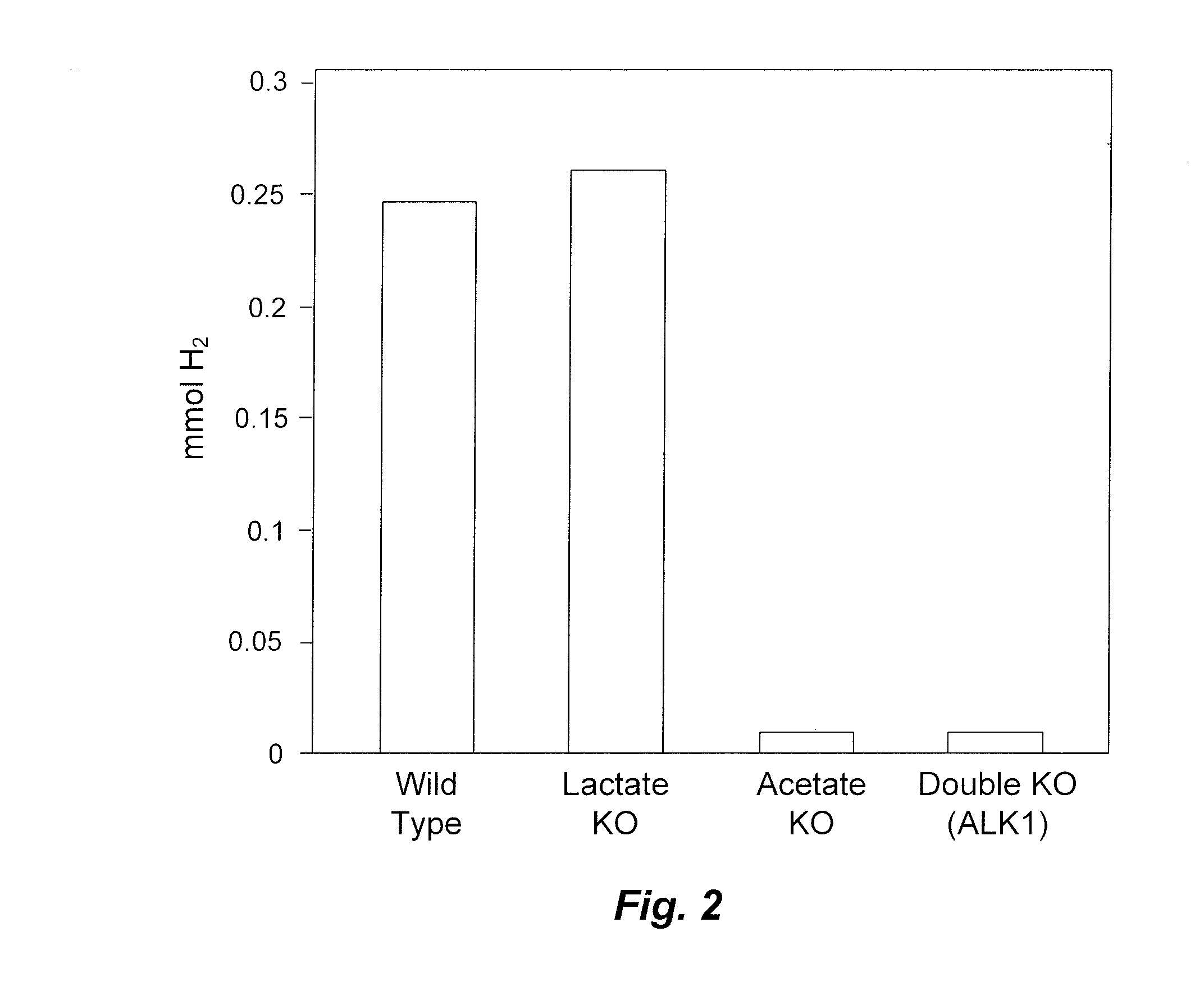
Mutant thermophilic organisms that consume a variety of biomass derived substrates are disclosed herein. Strains of Thermoanaerobacterium saccharolyticum with acetate kinase and phosphotransacetylase expression eliminated are disclosed herein. Further, strain ALK1 has been engineered by site directed homologous recombination to knockout both acetic acid and lactic acid production. Continuous culture involving a substrate concentration challenge lead to evolution of ALK1, and formation of a more robust strain designated ALK2. The organisms may be utilized for example in thermophilic SSF and SSCF reactions performed at temperatures that are optimal for cellulase activity to produce near theoretical ethanol yields without expressing pyruvate decarboxylase.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
XZ-A26 bacterial strain for producing L-alanine with high yield as well as construction method and application of XZ-A26 bacterial strain
Owner:ANHUI HUAHENG BIOTECH
Novel rumen bacteria variants and process for preparing succinic acid employing the same
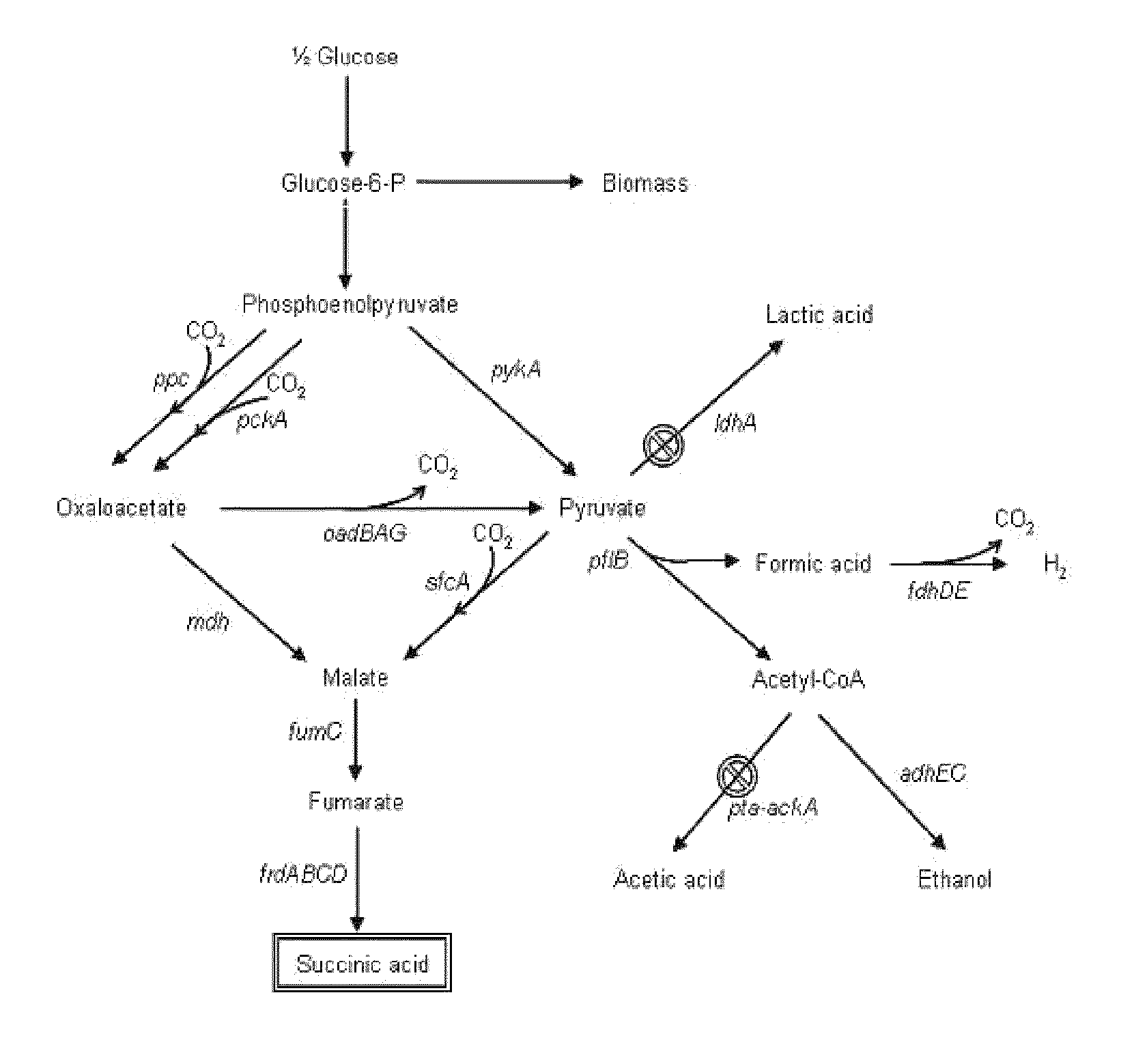
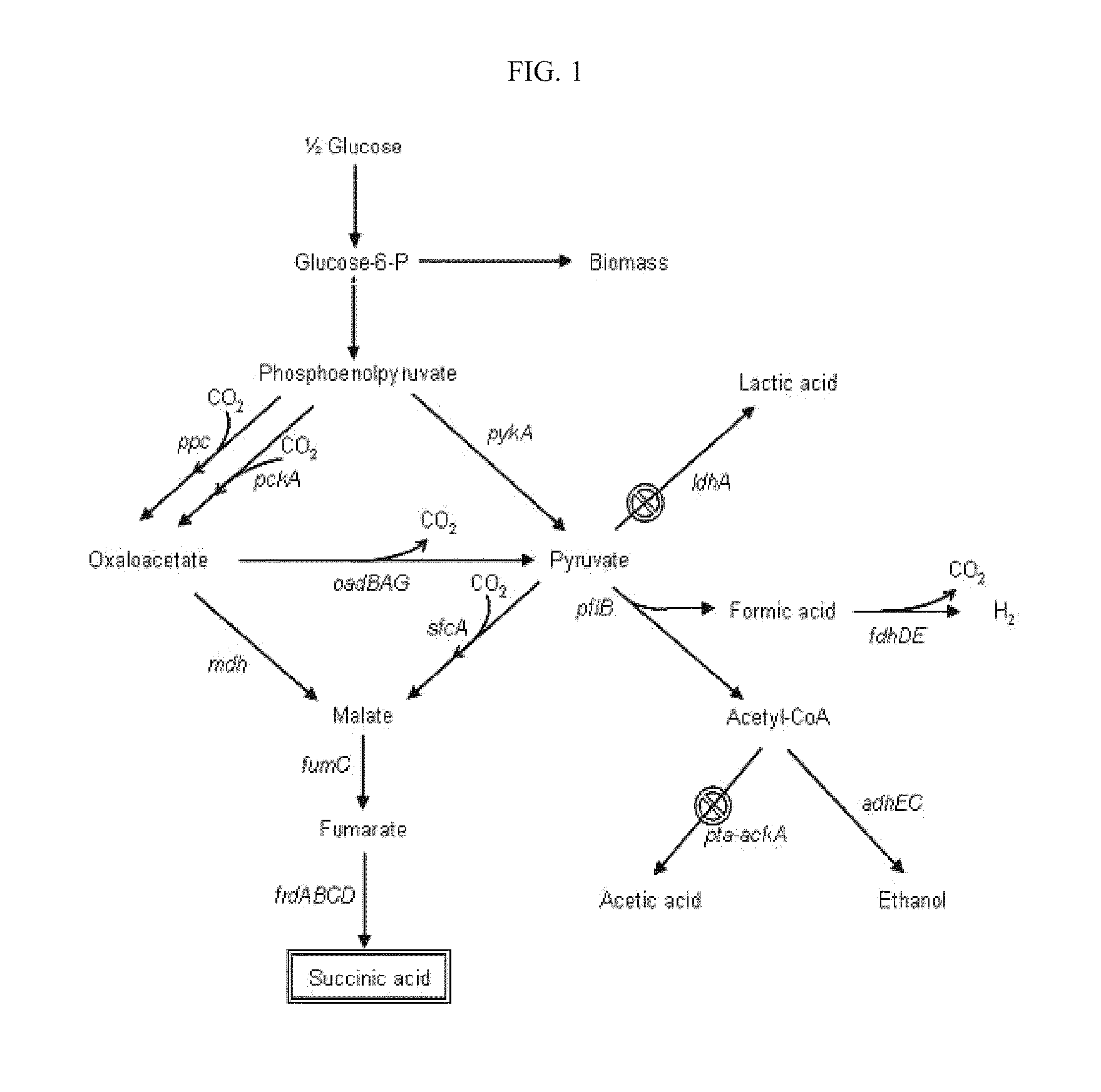
ActiveUS20070054387A1High yieldBacteriaSugar derivativesHigh concentrationPhosphoenolpyruvate carboxylase
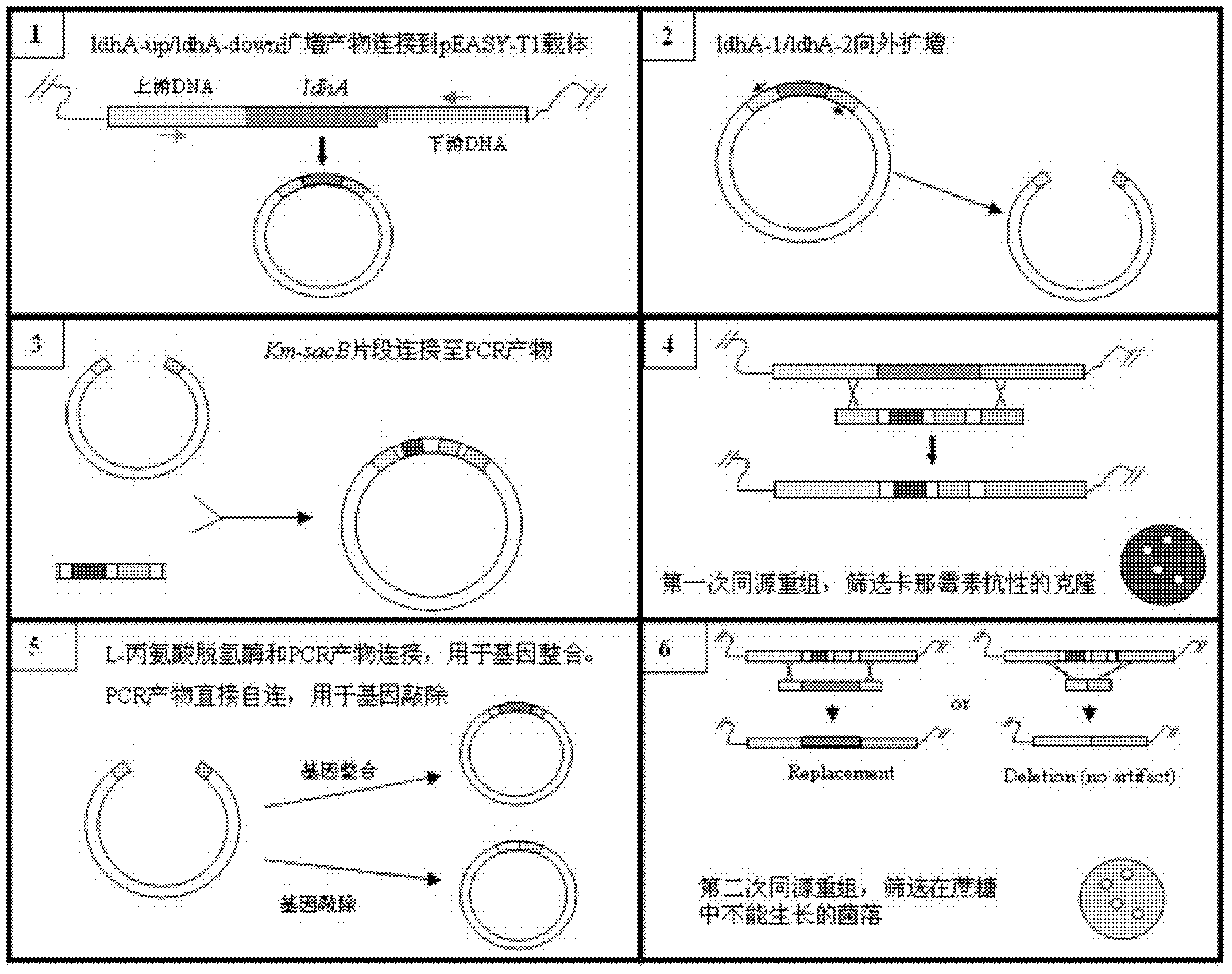
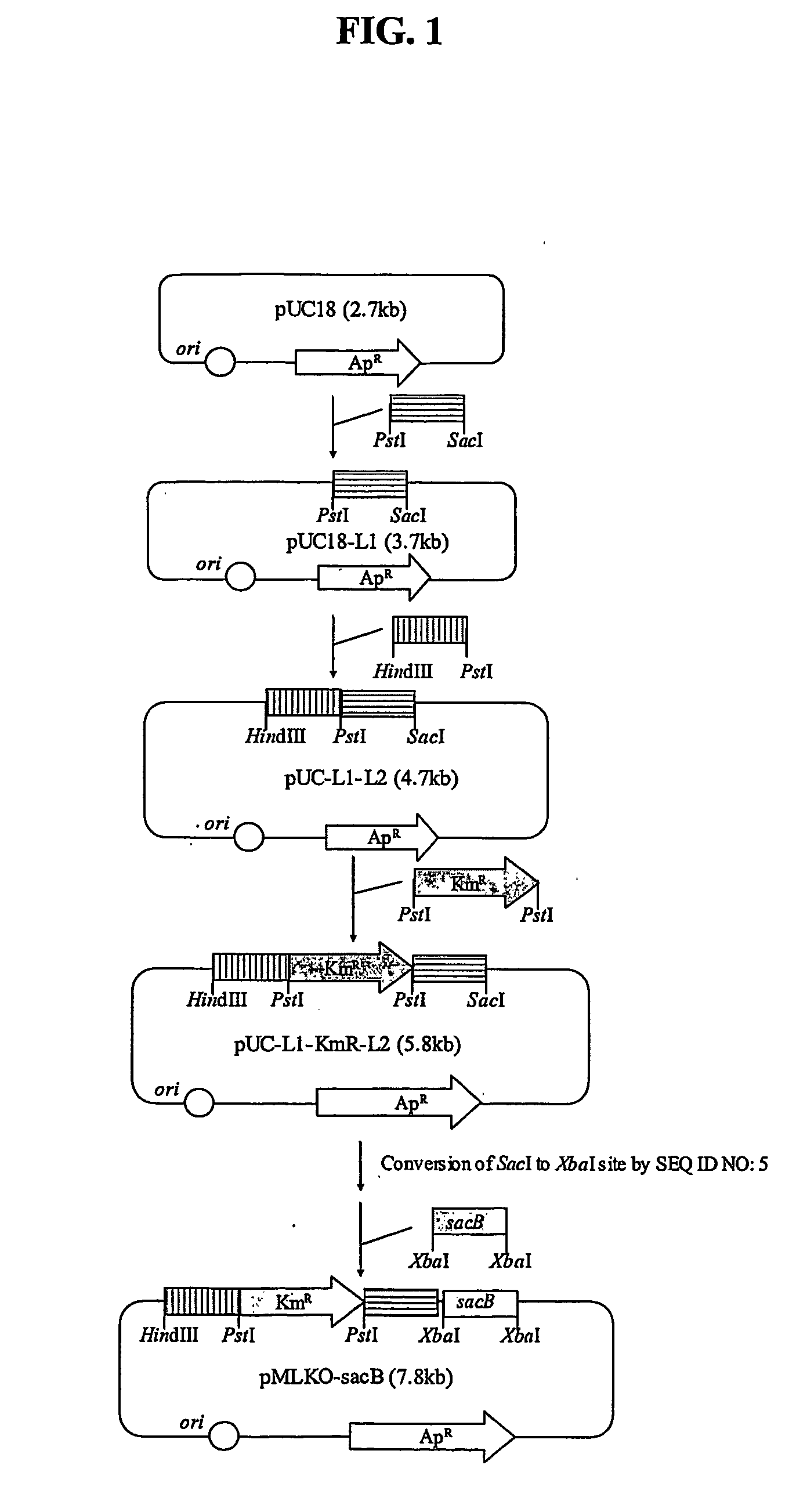
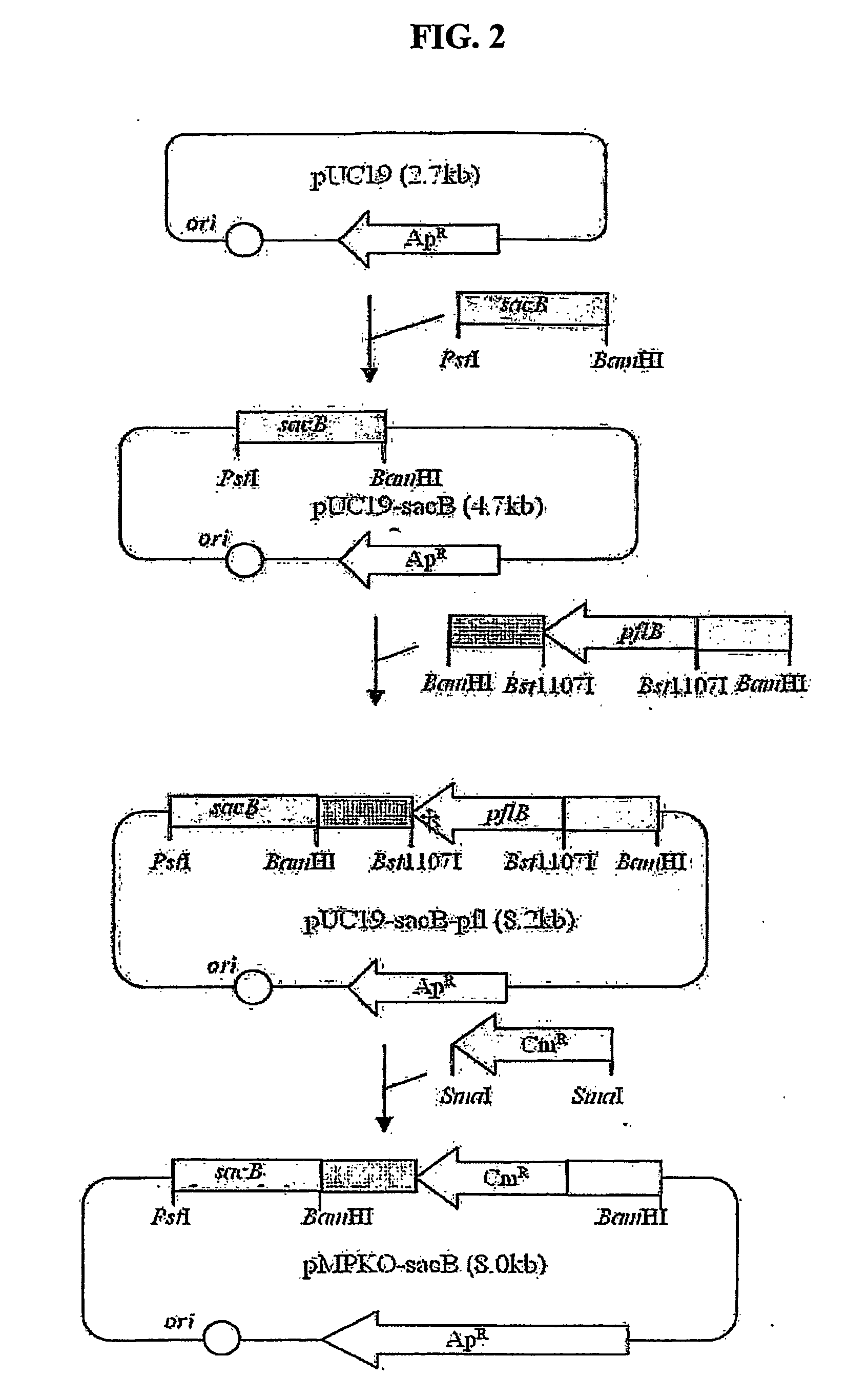
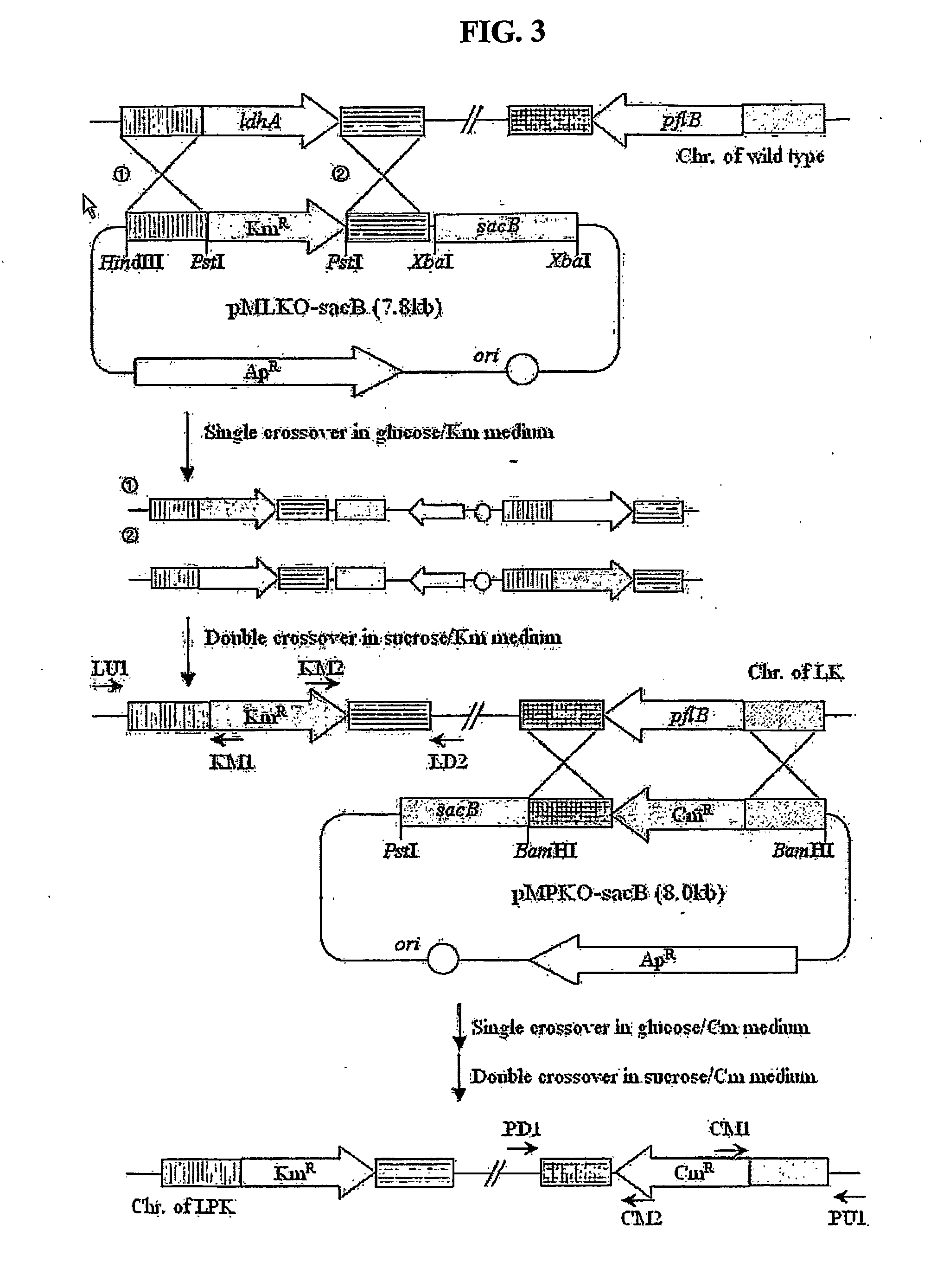
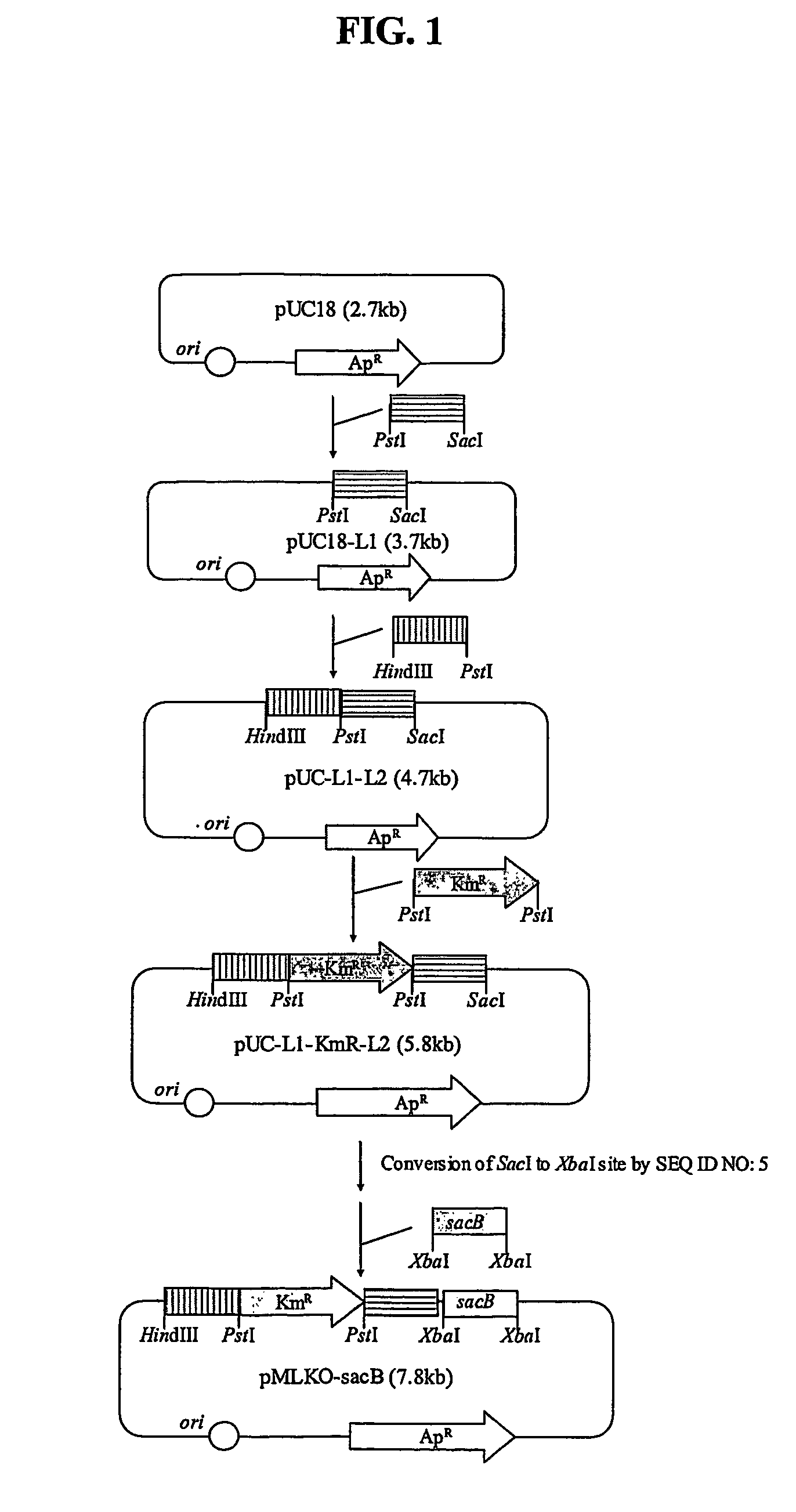
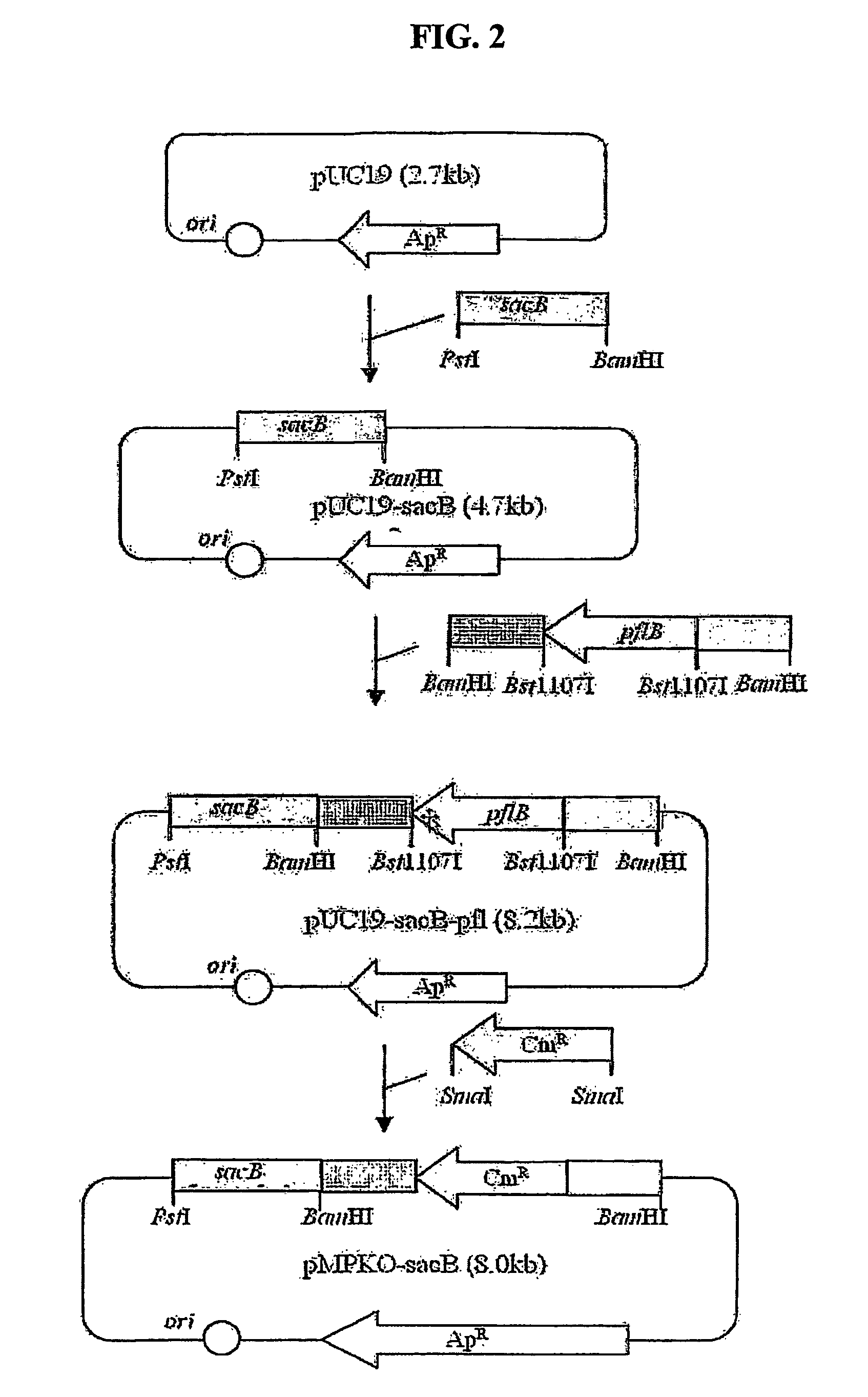
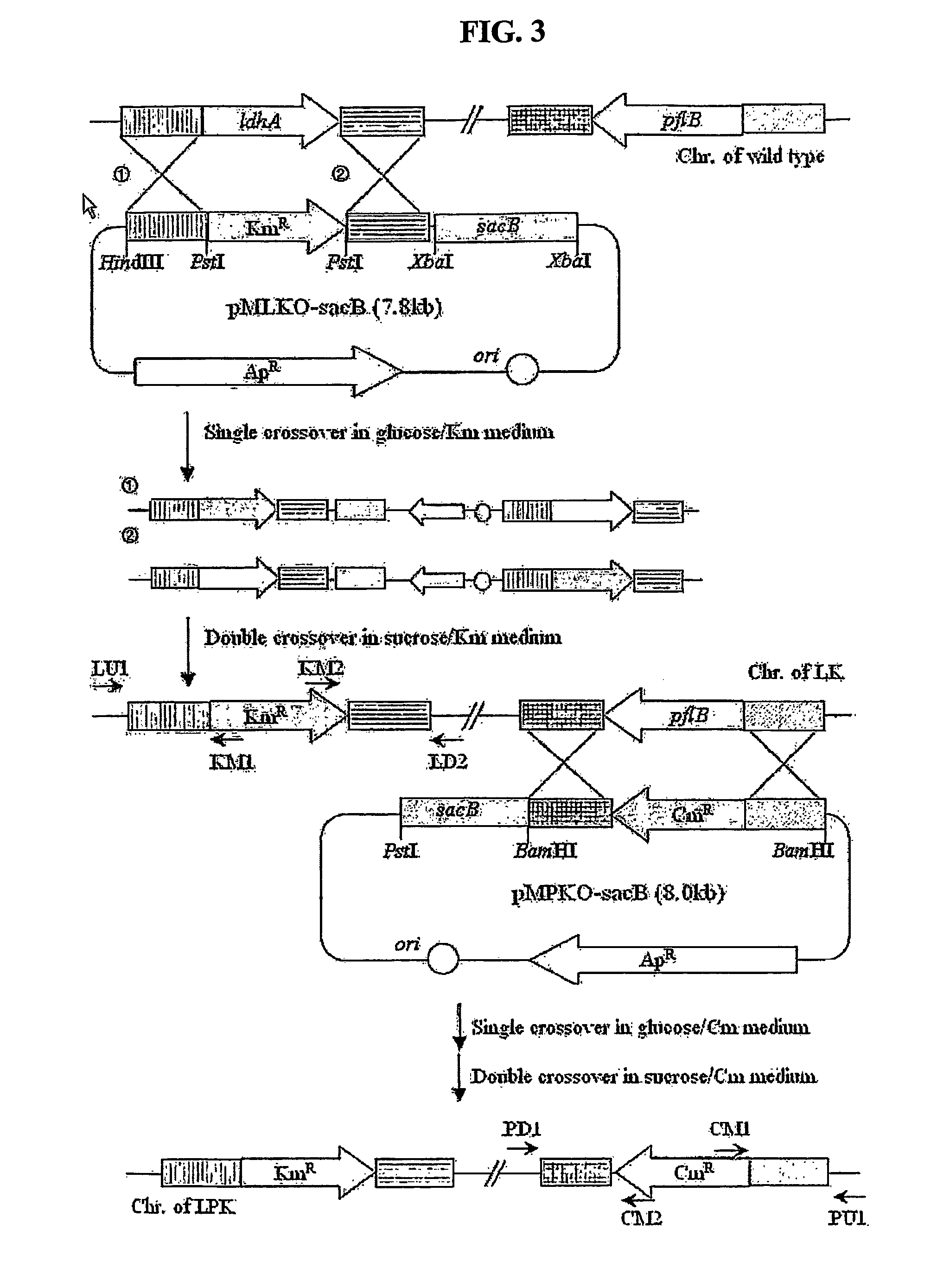
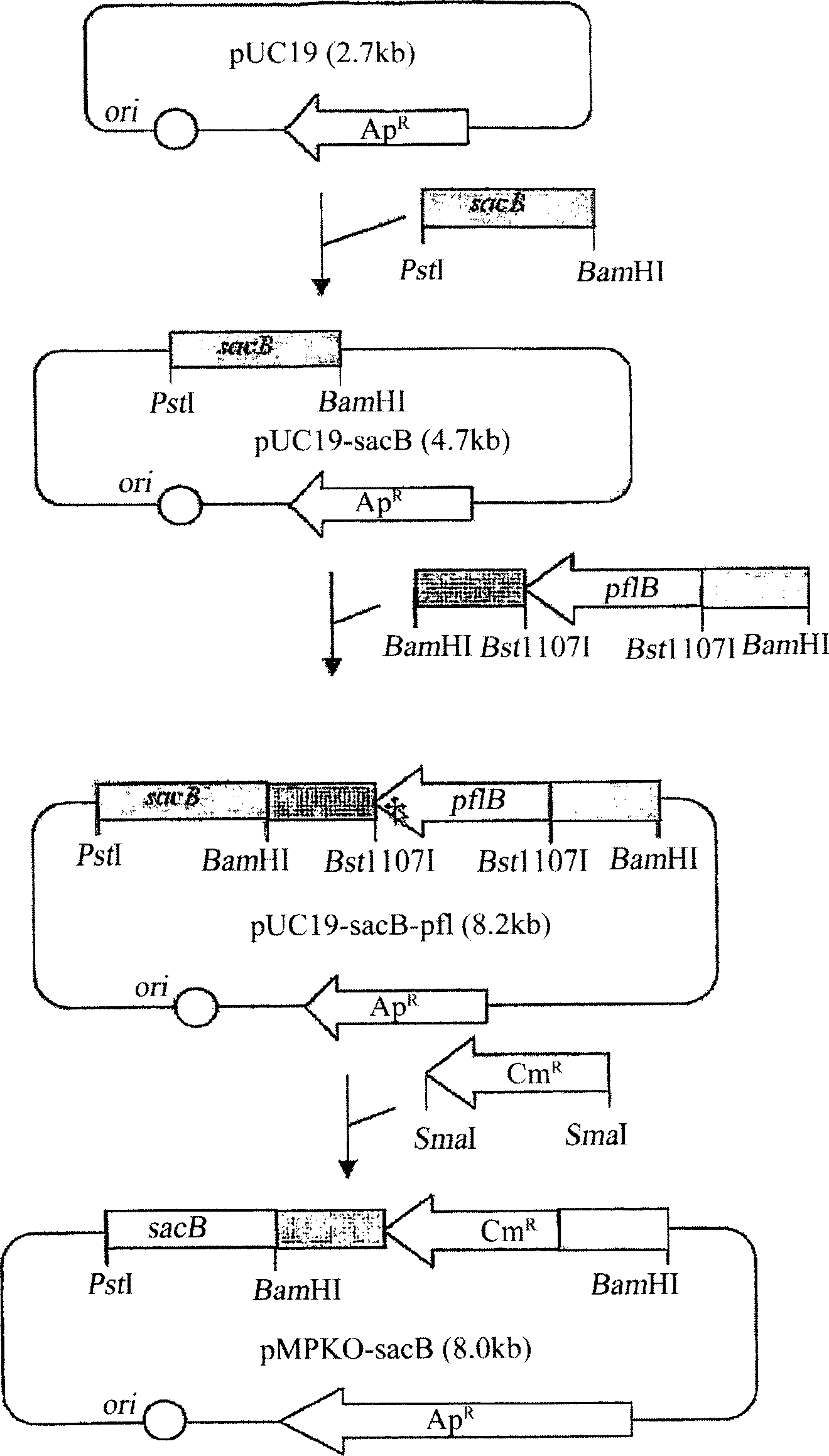
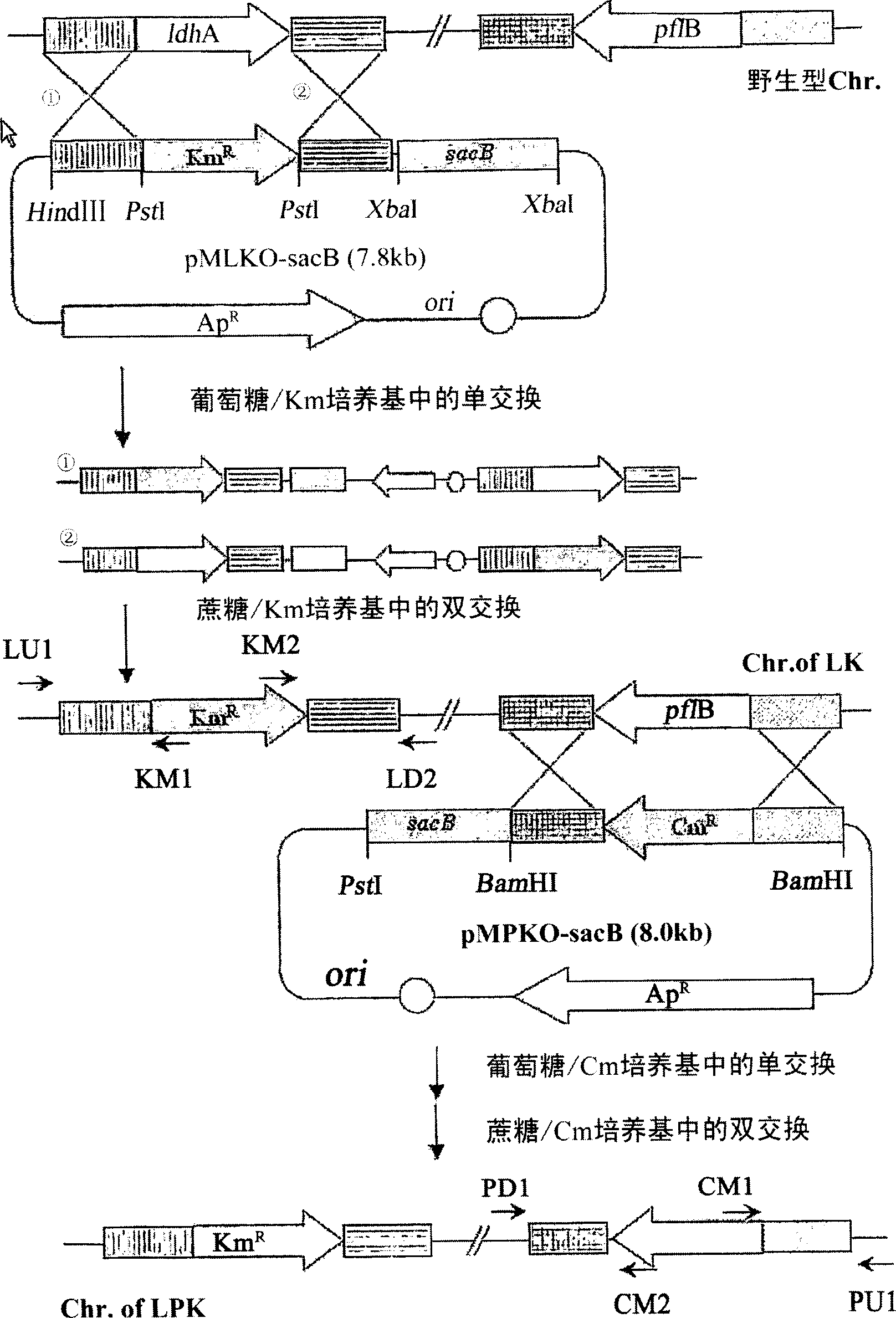
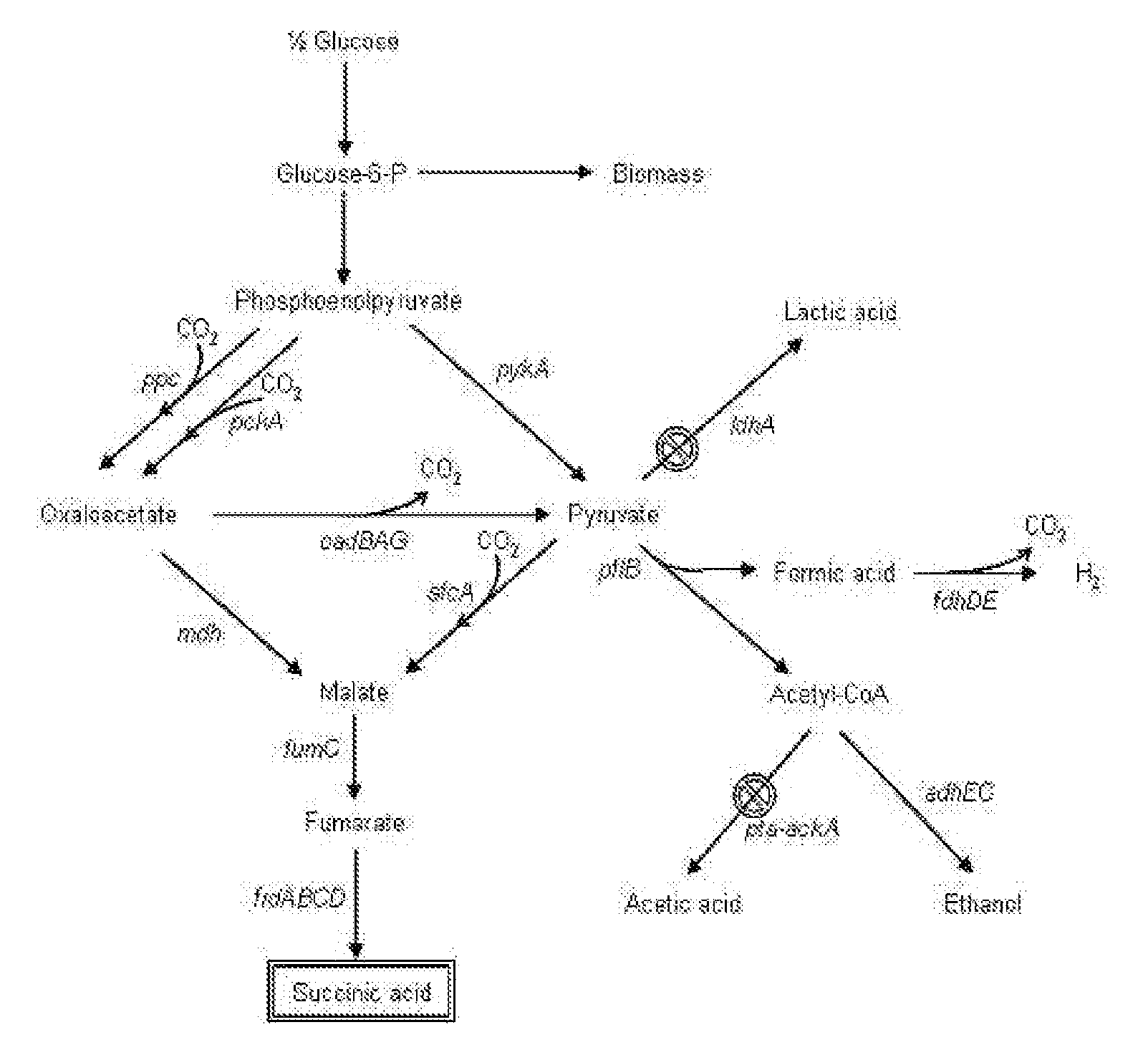
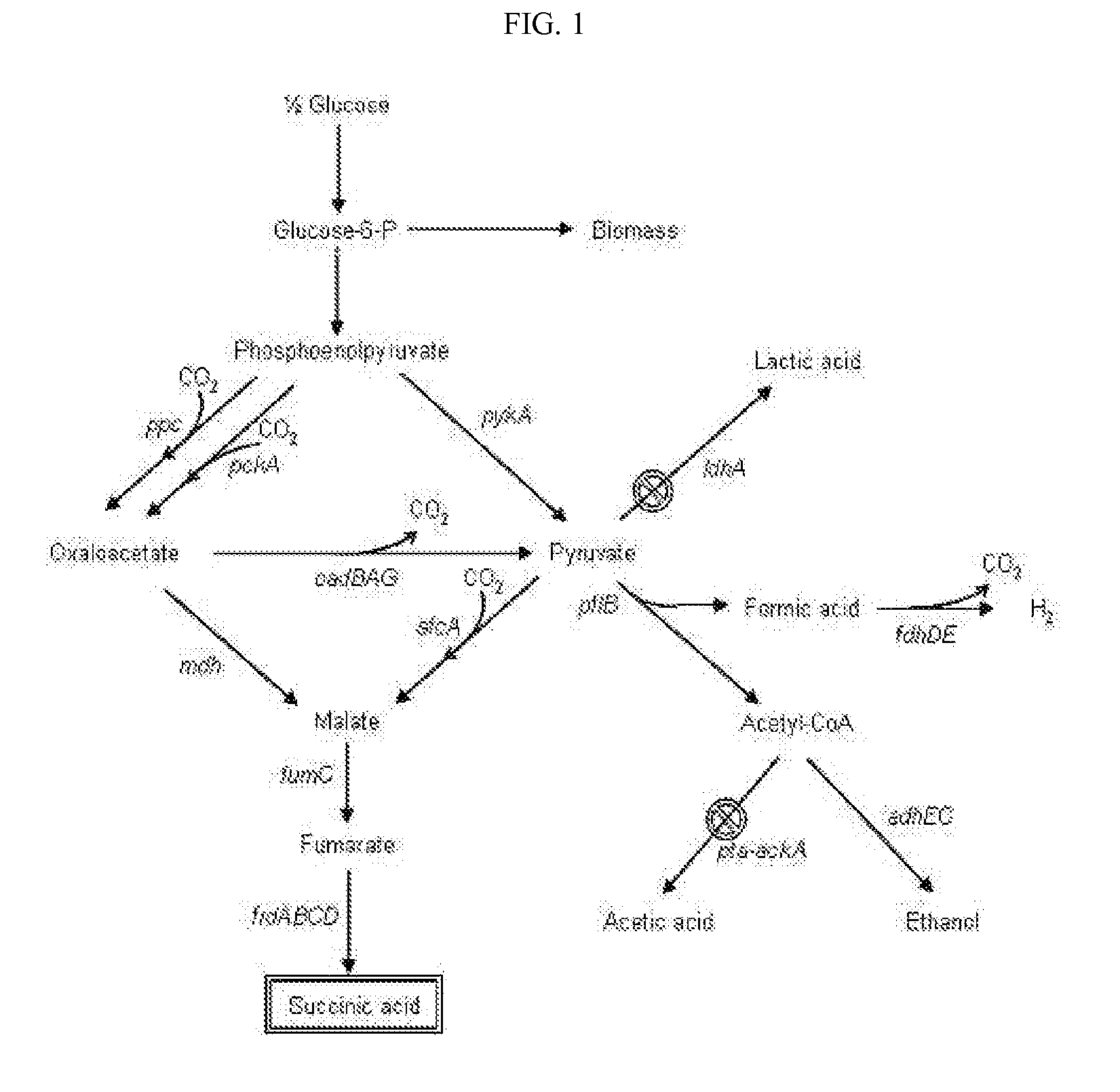
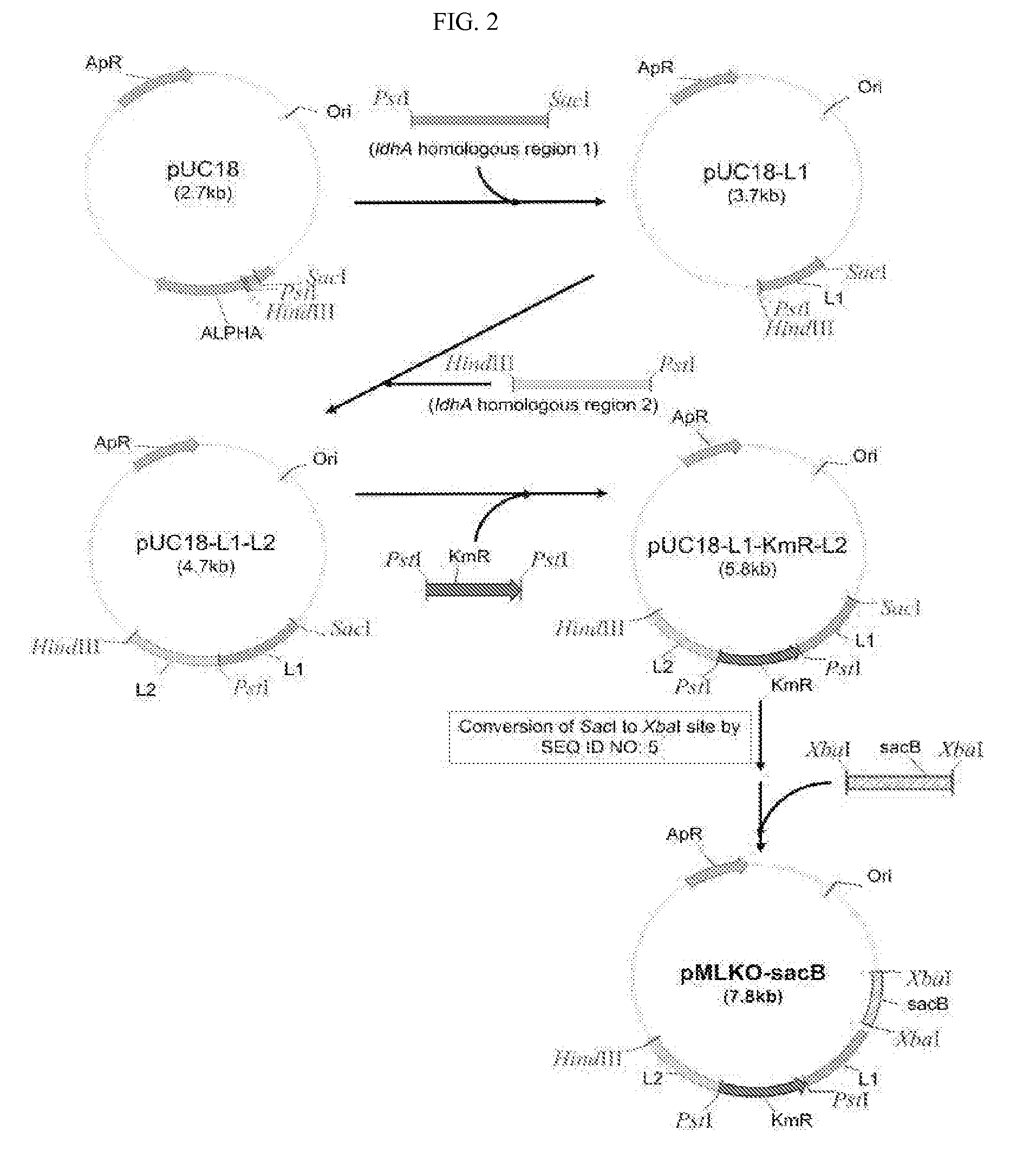
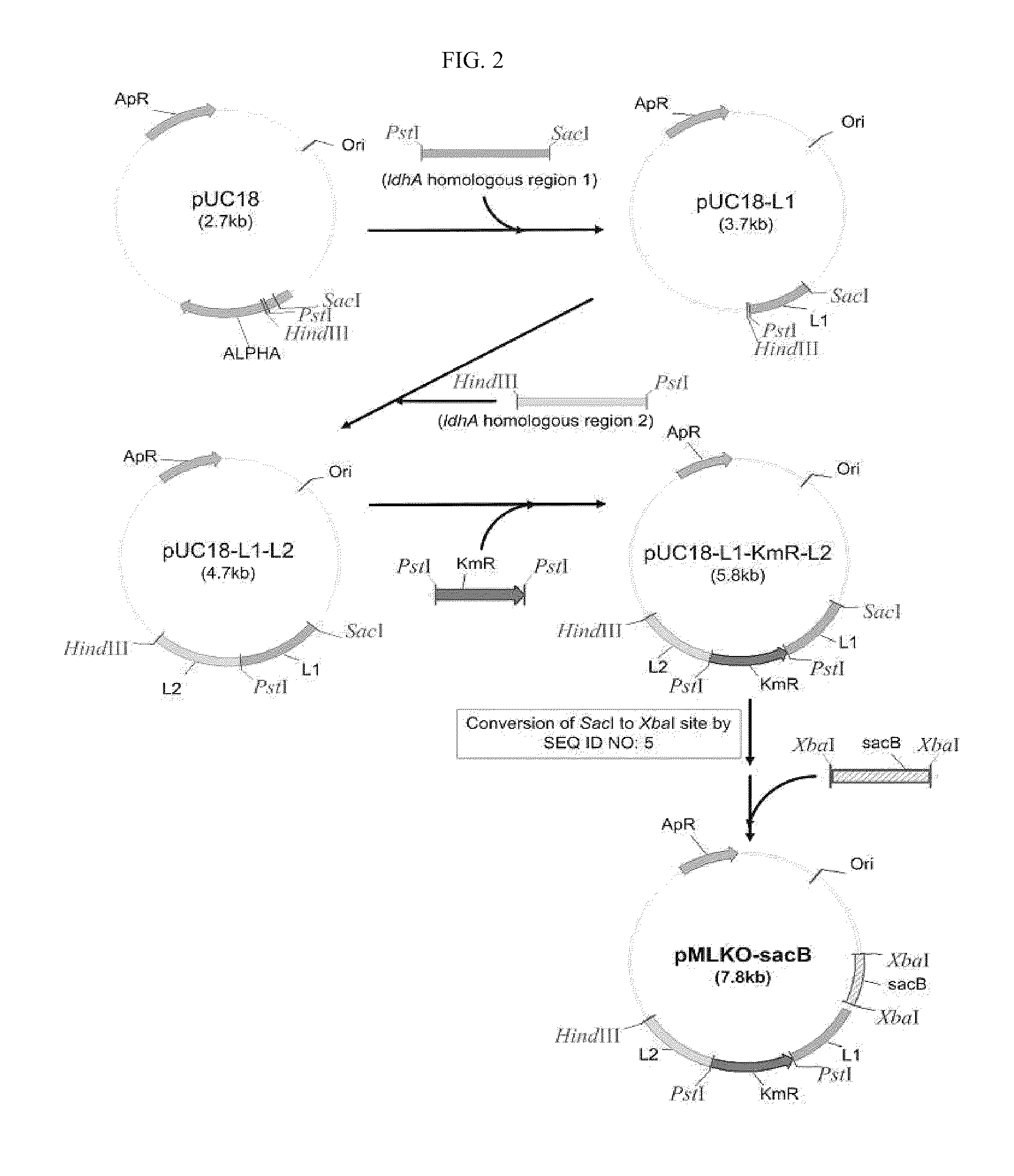
The present invention relates to novel rumen bacterial mutants resulted from the disruption of a lactate dehydrogenase gene (ldhA) and a pyruvate formate-lyase gene (pfl) (which are involved in the production of lactic acid, formic acid and acetic acid) from rumen bacteria; a novel bacterial mutant (Mannheimia sp. LPK7) having disruptions of a lactate dehydrogenase gene (ldhA), a pyruvate formate-lyase gene (pfl), a phosphotransacetylase gene (pta), and a acetate kinase gene (ackA); a novel bacterial mutant (Mannheimia sp. LPK4) having disruptions of a lactate dehydrogenase gene (ldhA), a pyruvate formate-lyase gene (pfl) and a phosphoenolpyruvate carboxylase gene (ppc) involved in the immobilization of CO2 in a metabolic pathway of producing succinic acid; and a method for producing succinic acid, which is characterized by the culture of the above mutants in anaerobic conditions. The inventive bacterial mutants have the property of producing succinic acid at high concentration while producing little or no organic acids, as compared to the prior wild-type strains of producing various organic acids. Thus, the inventive bacterial mutants are useful as strains for the industrial production of succinic acid.
Owner:KOREA ADVANCED INST OF SCI & TECH
Rumen bacteria variants and process for preparing succinic acid employing the same
ActiveUS7470530B2High yieldSugar derivativesBacteriaHigh concentrationPhosphoenolpyruvate carboxylase
Provided are novel rumen bacterial mutants resulted from the disruption of a lactate dehydrogenase gene (ldhA) and a pyruvate formate-lyase gene (pfl) from rumen bacteria; a novel bacterial mutant (Mannheimia sp. LPK7) having disruptions of a ldhA, a pfl,a phosphotransacetylase gene (pta), and a acetate kinase gene (ackA); a novel bacterial mutant (Mannheimia sp. LPK4) having disruptions of a ldhA, a pfl, and a phosphoenolpyruvate carboxylase gene (ppc) involved in the immobilization of CO2 in a metabolic pathway of producing succinic acid; and a method for producing succinic acid, characterized by culture of the above mutants in anaerobic conditions. The bacterial mutants have the property of producing succinic acid at high concentration while producing little or no organic acids, as compared to the prior wild-type strains of producing various organic acids. Thus, the bacterial mutants are useful as strains for the industrial production of succinic acid.
Owner:KOREA ADVANCED INST OF SCI & TECH
Engineering bacteria producing DL-alanine and method of producing DL-alanine by using engineering bacteria
ActiveCN103045528AIncrease productionHigh yieldBacteriaMicroorganism based processesEthanol dehydrogenaseAlanine racemase
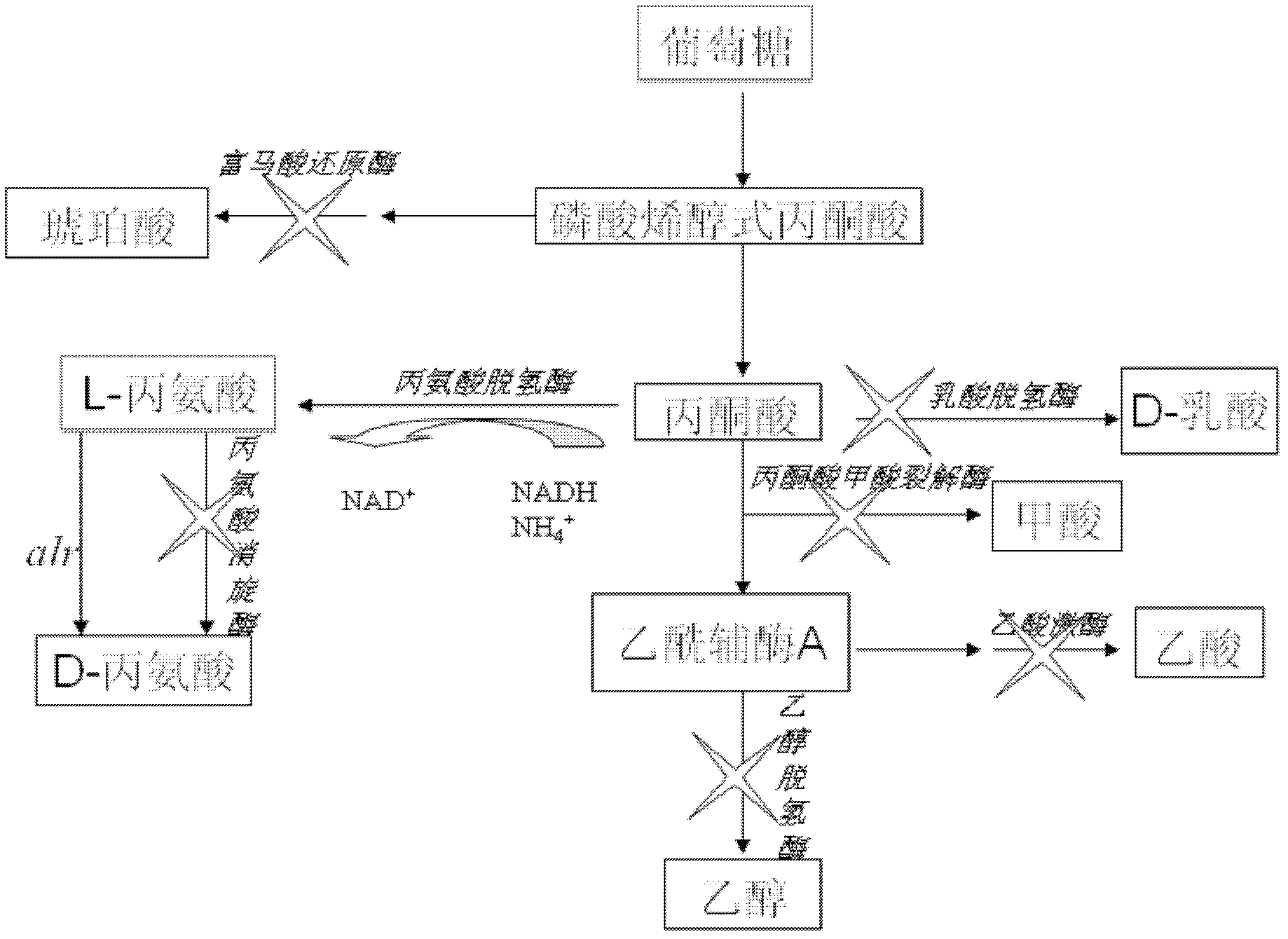
The invention discloses a strain of engineering bacteria producing DL-alanine. Lactic dehydrogenase, pyruvate formate lyase, alcohol dehydrogenase, acetic acid kinase, fumaric acid reductase, alanine racemase and methyl glyoxal synthetase of the strain of engineering bacteria producing the DL-alanine are inactivated; and exogenous L-alanine dehydrogenase gene and alanine racemase gene are integrated on the chromosome of the engineering bacteria. According to the invention, pyroracemic acid, an intermediate product of the glycolysis is converted to L-alanine by integrating the exogenous L-alanine dehydrogenase gene into the chromosome of the engineering bacteria; and an exogenous alanine racemase gene is further integrated into the chromosome, and part of the L-alanine is converted into D-alanine. Then producing the DL-alanine from raw material sugar in one step is realized, the production period of the DL-alanine is decreased and the productivity of the DL-alanine is enhanced.
Owner:ANHUI HUAHENG BIOTECH
Method for synthesizing glutathione by enzymatic catalysis
ActiveCN106086126AHigh synthesis efficiencyShort synthesis timePeptidesFermentationATP regenerationGlutamic acid
The present invention discloses a method for synthesizing glutathione by enzymatic catalysis, comprising the following steps: S1, mixing Upsilon-glutamylcysteine synthetase liquid and glutathione synthetase liquid to obtain mixed liquid A, mixing the mixed liquid A with acetate kinase to obtain mixed liquid B; S2, adding an immobilized carrier in the mixed liquid B, stirring for immobilizing, and filtering to obtain an immobilized enzyme; S3, blending glutamic acid, L-cysteine, glycine, magnesium sulfate, ATP (adenosine triphosphate) and acetyl phosphate into a reaction liquid, adding the immobilized enzyme, and stirring for reacting; S4, after reacting, filtering the reaction liquid, and extracting and refining filtrate to obtain the glutathione. The method of the invention has high synthetic efficiency, the production content of the reaction liquid is higher than 10 g / L, and substrate conversion rate reaches higher than 90%; by introducing acetate kinase to construct an ATP regeneration and coupling system, ATP charge is greatly reduced and production cost is significantly reduced; the immobilized enzyme is reusable and highly stable.
Owner:KAIPING GENUINE BIOCHEM PHARMA
Thermophillic Organisms For Conversion Of Lignocellulosic Biomass To Ethanol
Mutant thermophilic organisms that consume a variety of biomass derived substrates are disclosed herein. Strains of Thermoanaerobacterium saccharolyticum with acetate kinase and phosphotransacetylase expression eliminated are disclosed herein. Further, strain ALK1 has been engineered by site directed homologous recombination to knockout both acetic acid and lactic acid production. Continuous culture involving a substrate concentration challenge lead to evolution of ALK1, and formation of a more robust strain designated ALK2. Both organisms produce near theoretical ethanol yields without expressing pyruvate decarboxylase
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Method of preparing creatine phosphate sodium
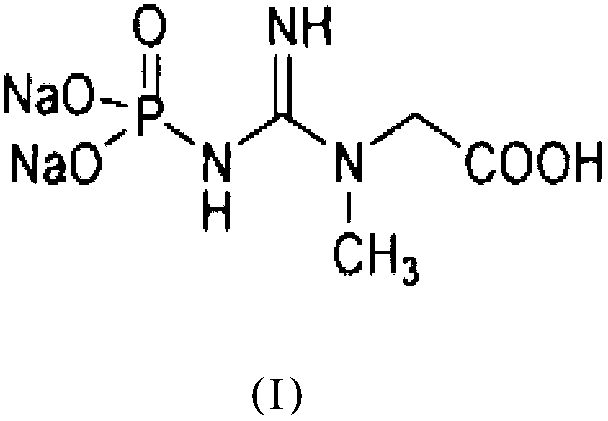
The invention relates to a method of preparing creatine phosphate sodium by utilizing a gene engineering technology. The method comprises the steps: respectively constructing creatine kinase and acetate kinase expression strains, removing the limit of creatine kinase-containing animal source, specially purifying the creatine kinase and acetate kinase by utilizing an affinity purification technology, co-immobilizing the creatine kinase and acetate kinase onto vector particles to form co-immobilized enzyme, continuously regenerating ATP from the acetate kinase for sustained reaction while the creatine kinase catalyzes and generates creatine phosphate sodium, thereby finally providing low-cost, green and pollution-free creatine phosphate sodium.
Owner:HUNAN BAOLISHI BIOTECH
Novel rumen bacteria variants and process for preparing succinic acid employing the same
The present invention relates to novel rumen bacterial mutants resulted from the disruption of a lactate dehydrogenase gene (ldhA) and a pyruvate formate-lyase gene (pfl) (which are involved in the production of lactic acid, formic acid and acetic acid) from rumen bacteria; a novel bacterial mutant (Mannheimia sp. LPK7) having disruptions of a lactate dehydrogenase gene (ldhA), a pyruvate formate-lyase gene (pfl), a phosphotransacetylase gene (pta), and a acetate kinase gene (ackA); a novel bacterial mutant (Mannheimia sp. LPK4) having disruptions of a lactate dehydrogenase gene (ldhA), a pyruvate formate-lyase gene (pfl) and a phosphoenolpyruvate carboxylase gene (ppc) involved in the immobilization of CO2 in a metabolic pathway of producing succinic acid; and a method for producing succinic acid, which is characterized by the culture of the above mutants in anaerobic conditions. The inventive bacterial mutants have the property of producing succinic acid at high concentration while producing little or no organic acids, as compared to the prior wild-type strains of producing various organic acids. Thus, the inventive bacterial mutants are useful as strains for the industrial production of succinic acid.
Owner:KOREA ADVANCED INST OF SCI & TECH
Novel engineered microorganism producing homo-succinic acid and method for preparing succinic acid using the same
ActiveUS20090203095A1High yieldIncrease productivityBacteriaOther foreign material introduction processesHigh concentrationMannheimia
The present invention relates to a mutant microorganism, which is selected from the group consisting of genus Mannheimia, genus Actinobacillus and genus Anaerobiospirillum, producing homo-succinic acid and a method for producing homo-succinic acid using the same, and more particularly to a mutant microorganism producing succinic acid at a high concentration while producing little or no other organic acids in anaerobic conditions, which is obtained by disrupting a gene encoding lactate dehydrogenase (ldhA), a gene encoding phosphotransacetylase (pta), and a gene encoding acetate kinase (ackA), without disrupting a gene encoding pyruvate formate lyase (pfl), as well as a method for producing succinic acid using the same. The inventive mutant microorganism has the property of having a high growth rate and succinic acid productivity while producing little or no organic acids, as compared to the prior strains producing succinic acid. Thus, the inventive mutant microorganism is useful to produce succinic acid for industrial use.
Owner:KOREA ADVANCED INST OF SCI & TECH
Genetic engineering bacterium for producing glycollic acid by acetic acid, and building method and application thereof
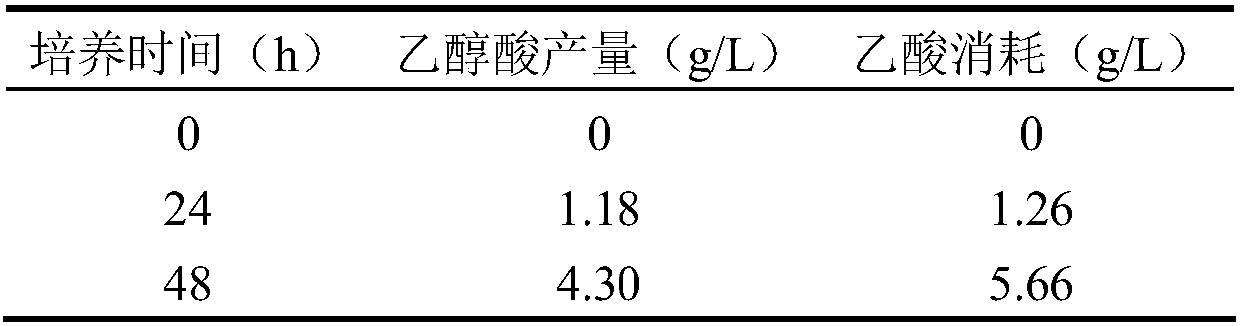
ActiveCN107603998AIncrease productionHigh yieldBacteriaMicroorganism based processesMalate synthaseAcetyl Coenzyme A Synthetase
The invention discloses a genetic engineering bacterium for producing glycollic acid by acetic acid, and a building method and application thereof. A method for preparing the genetic engineering bacterium for producing glycollic acid provided by the invention comprises the following steps of improving the expression and / or activity of acetylcoenzyme A synthetase, phosphotransacetylase, acetokinase, citrate synthase, isocitrate lyase, isocitrate dehydrogenase kinase and glyoxylate reductase in the recipient bacterium; reducing the expression and / or the activity of malate synthase, glycolate oxidase and isocitrate lyase repressor protein in the recipient bacterium, so that the engineering bacterium for producing the glycollic acid is obtained. The recipient bacterium is a bacterium capable of using acetic acid as a carbon source for growth. The prepared genetic engineering bacterium uses acetic acid as the carbon source for producing the glycollic acid; in addition, the production quantity and yield of the glycollic acid in shaking culture can reach the relatively high level; relatively good industrial application prospects are realized.
Owner:BEIJING UNIV OF CHEM TECH
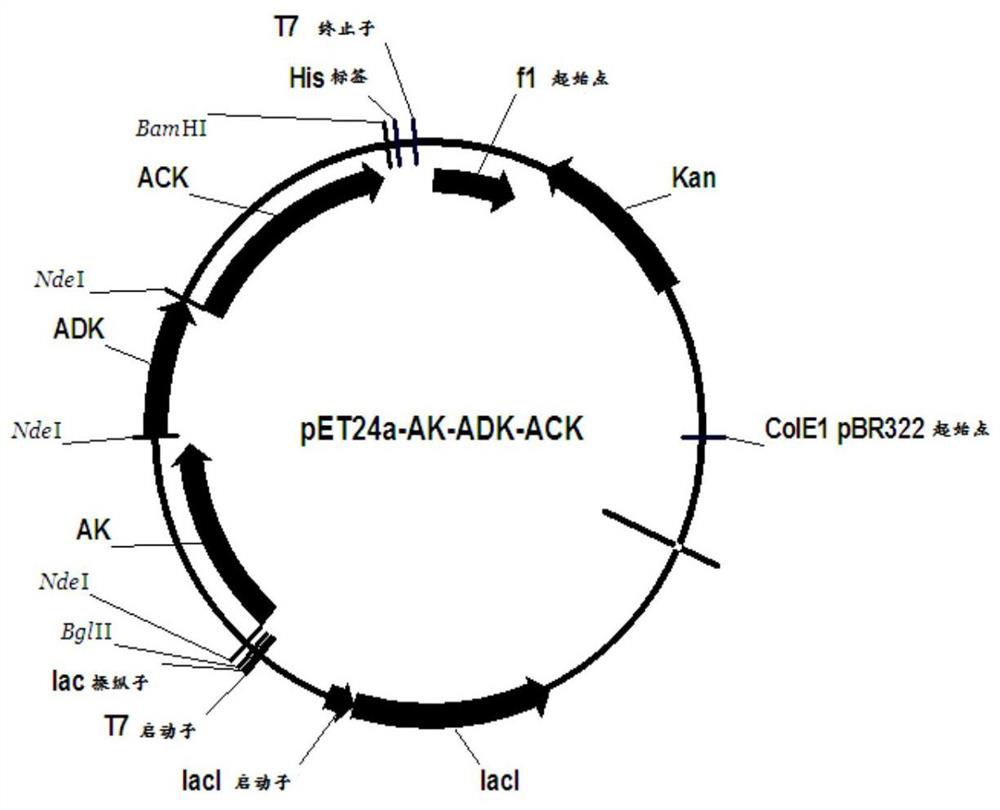
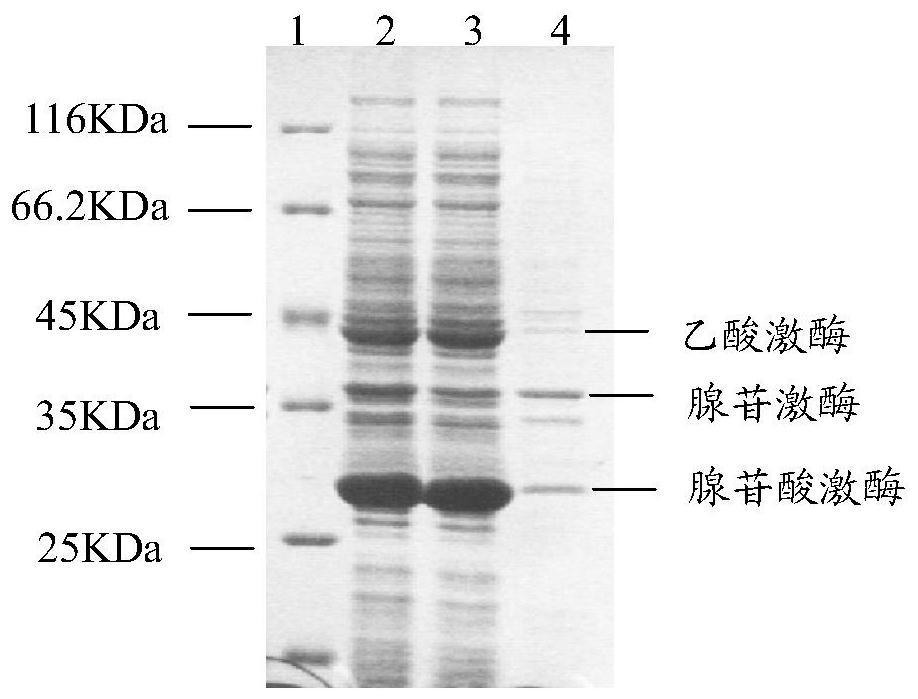
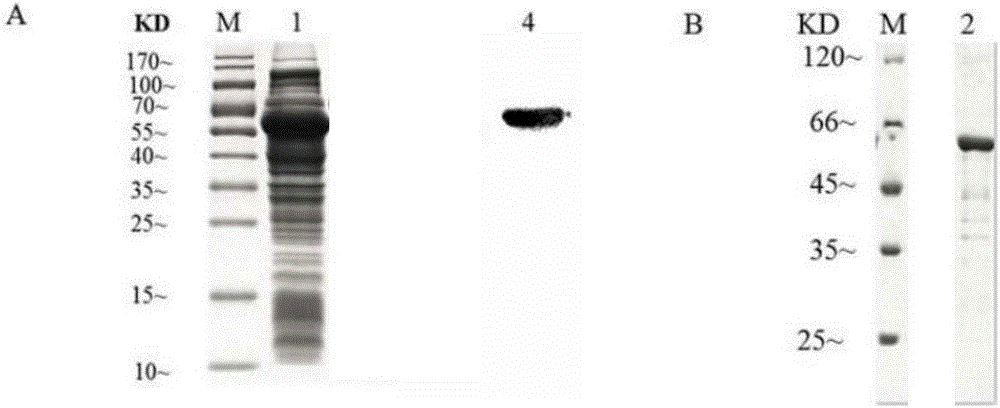
Recombinant expression vector, recombinant expression host and method for synthesizing ATP (adenosine triphosphoric acid) from recombinant expression host
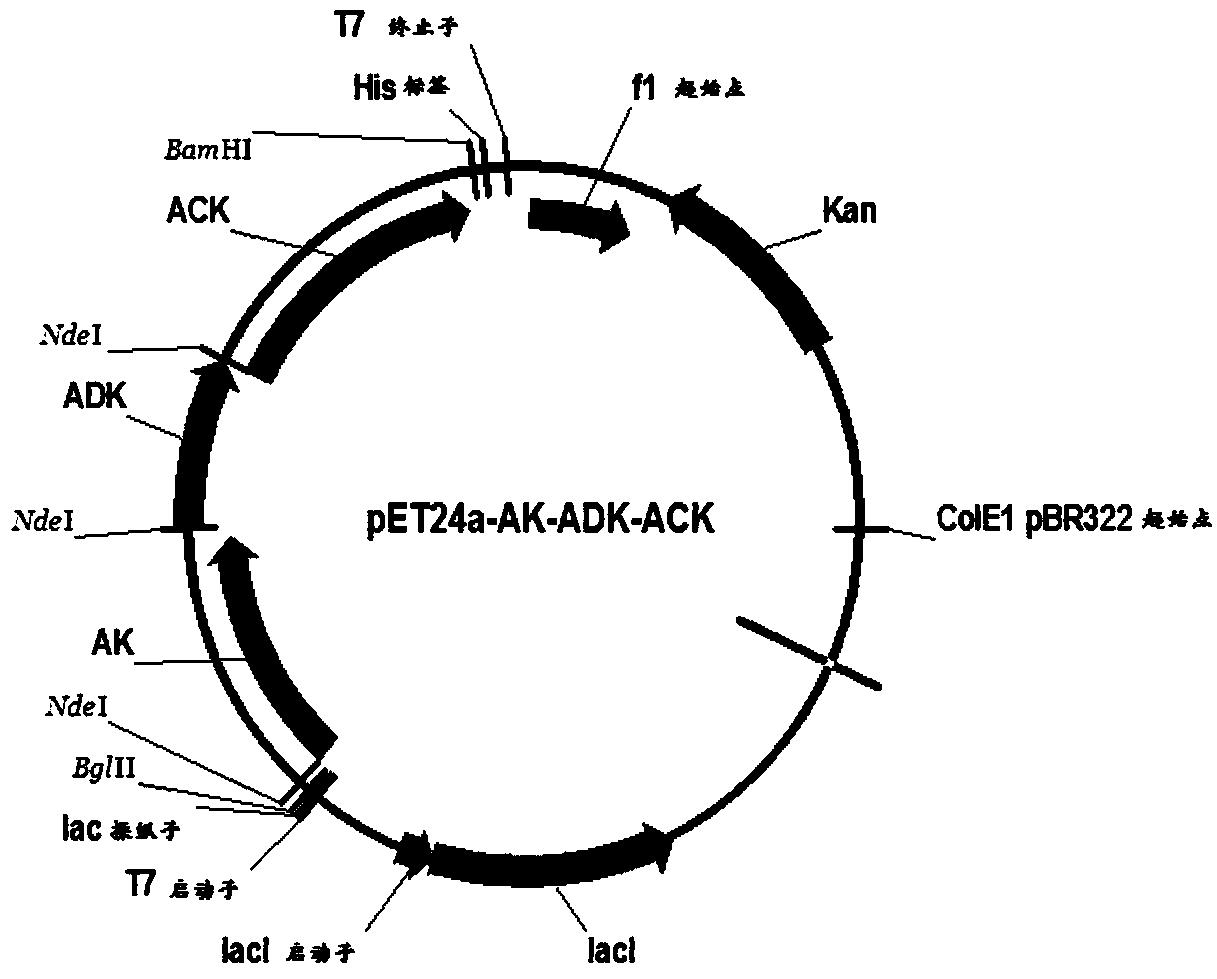
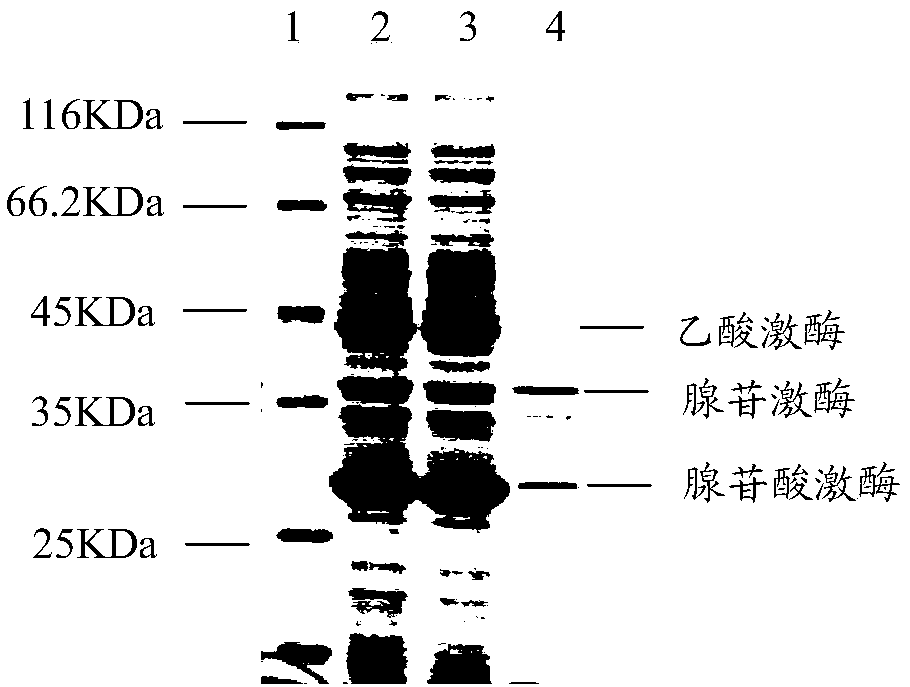
ActiveCN108753808AImprove conversion rateHigh yieldFermentationVector-based foreign material introductionAdenylate kinaseAdenosine
The invention belongs to the field of bioengineering, relates to preparation of ATP (adenosine triphosphoric acid), and provides a recombinant expression vector and a recombinant expression host containing the same. The recombinant expression vector contains polynucleotide of encoded adenosine kinase, adenylate kinase and acetokinase. The invention further provides a method for synthesizing ATP from the recombinant expression host. According to the invention, ATP is synthesized from the recombinant expression host by whole-cell catalysis, adenosine conversion rate, ATP yield and reaction efficiency are increased, and the reaction system contains simple components and is low in cost.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Genetic engineering bacteria for producing PHA (polyhydroxyalkanoates) from acetic acid and propionic acid as well as construction method and application of genetically engineering bacteria
ActiveCN108359628AReduce fermentation costsBacteriaMicroorganism based processesPropanoic acidAcetic acid ear
The invention discloses genetic engineering bacteria for producing PHA (polyhydroxyalkanoates) from acetic acid and propionic acid as well as a construction method and an application of the genetic engineering bacteria. The method for preparing the engineering bacteria for producing PHA comprises the following steps: improving expression and / or activity of acetokinase, phosphotransacetylase, PHA synthetase, beta-ketothiolase, acetoacetyl-CoA reductase, succinate hemiacetal dehydrogenase, 4-hydroxybutyrate dehydrogenase, 4-hydroxybutyl CoA:CoA-transferase and propionyl CoA transferase in recipient bacteria, and reducing expression and / or activity of non-CoA-cycle succinate hemiacetal dehydrogenase in the recipient bacteria to obtain the engineering bacteria for producing PHA, wherein the recipient bacteria can grow with acetic acid as a carbon source. The prepared engineering bacteria are used for producing PHA with acetic acid as the carbon source, PHA yield can reach a higher level, and the bacteria have better industrial application prospect. The genetic engineering bacteria have great application value.
Owner:BEIJING UNIV OF CHEM TECH
Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids
ActiveUS20160032323A1Reduce the amount of solutionHigh activityBacteriaHydrolasesHeterologousCitrate synthase
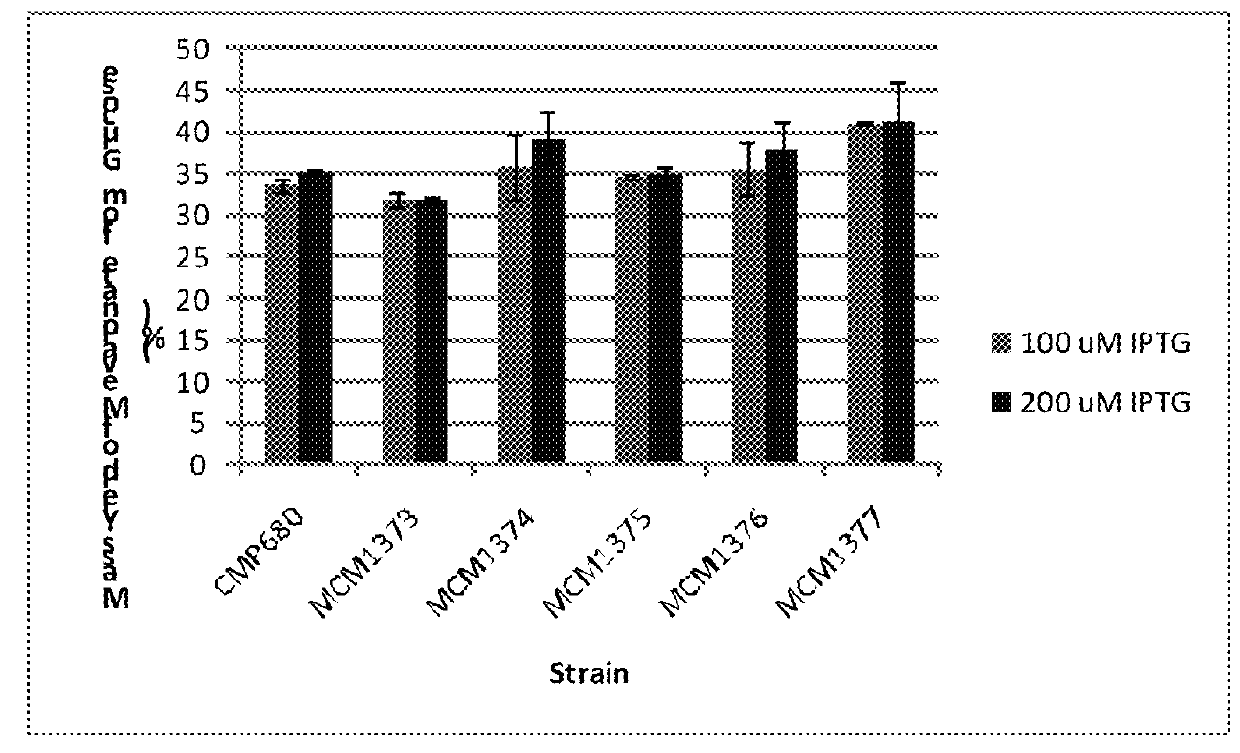
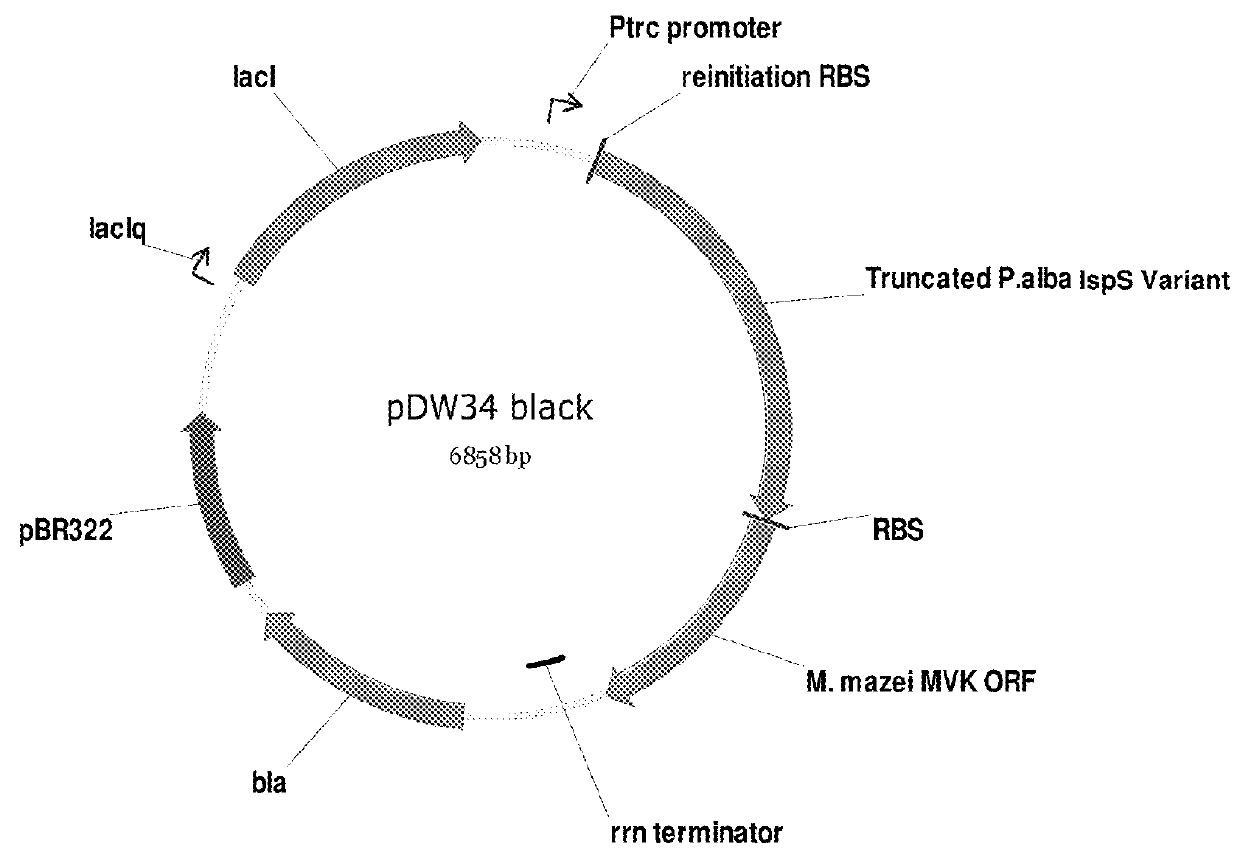
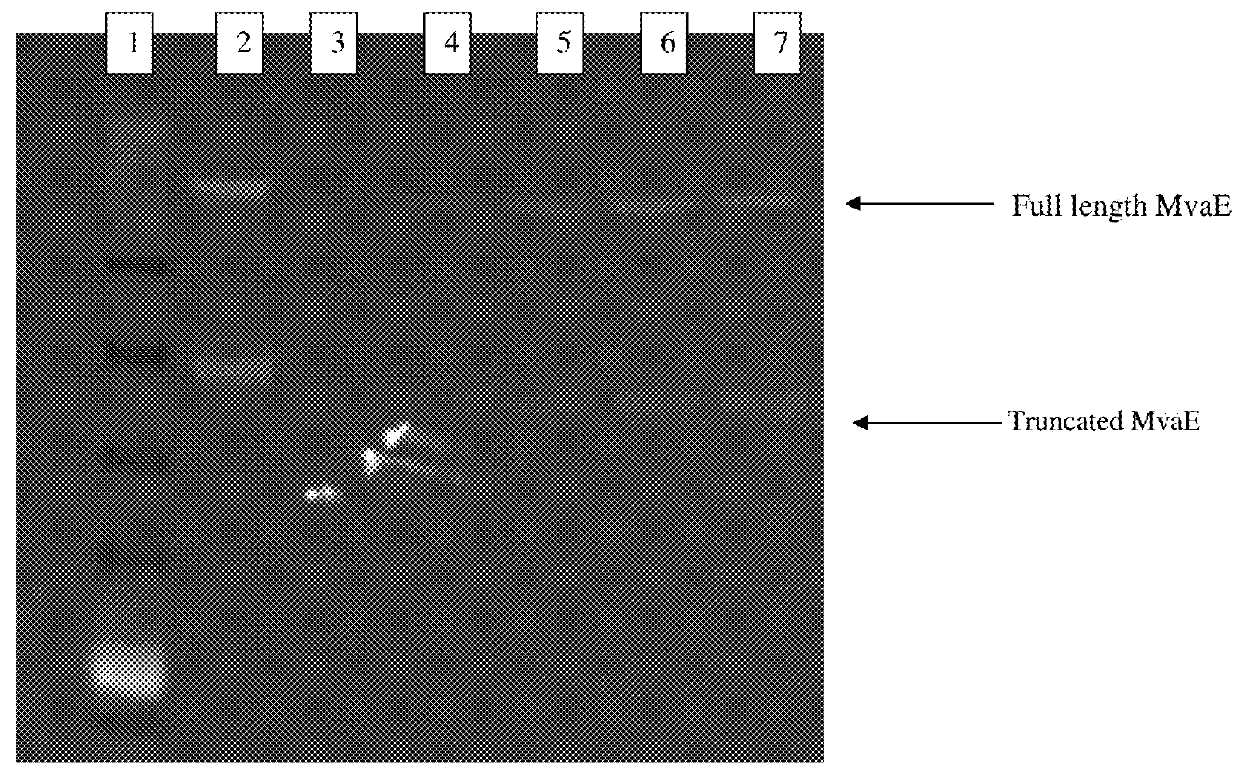
The invention features compositions and methods for the increased production of mevalonate, isoprene, isoprenoid precursor molecules, and / or isoprenoids in microorganisms by engineering a microorganism for increased carbon flux towards mevalonate production in the following enzymatic pathways: (a) citrate synthase, (b) phosphotransacetylase, (c) acetate kinase, (d) lactate dehydrogenase, (e) malic enzyme, and (f) pyruvate dehydrogenase such that one of more of the enzyme activity is modulated. In addition, production of mevalonate, isoprene, isoprenoid precursor molecules, and / or isoprenoids can be further enhanced by the heterologous expression of the mvaE and mvaS genes (such as, but not limited to, mvaE and mvaS genes from the organisms Listeria grayi DSM 20601, Enterococcus faecium, Enterococcus gallinarum EG2, and Enterococcus casseliflavus).
Owner:DANISCO US INC +1
XZ-A26 bacterial strain for producing L-alanine with high yield as well as construction method and application of XZ-A26 bacterial strain
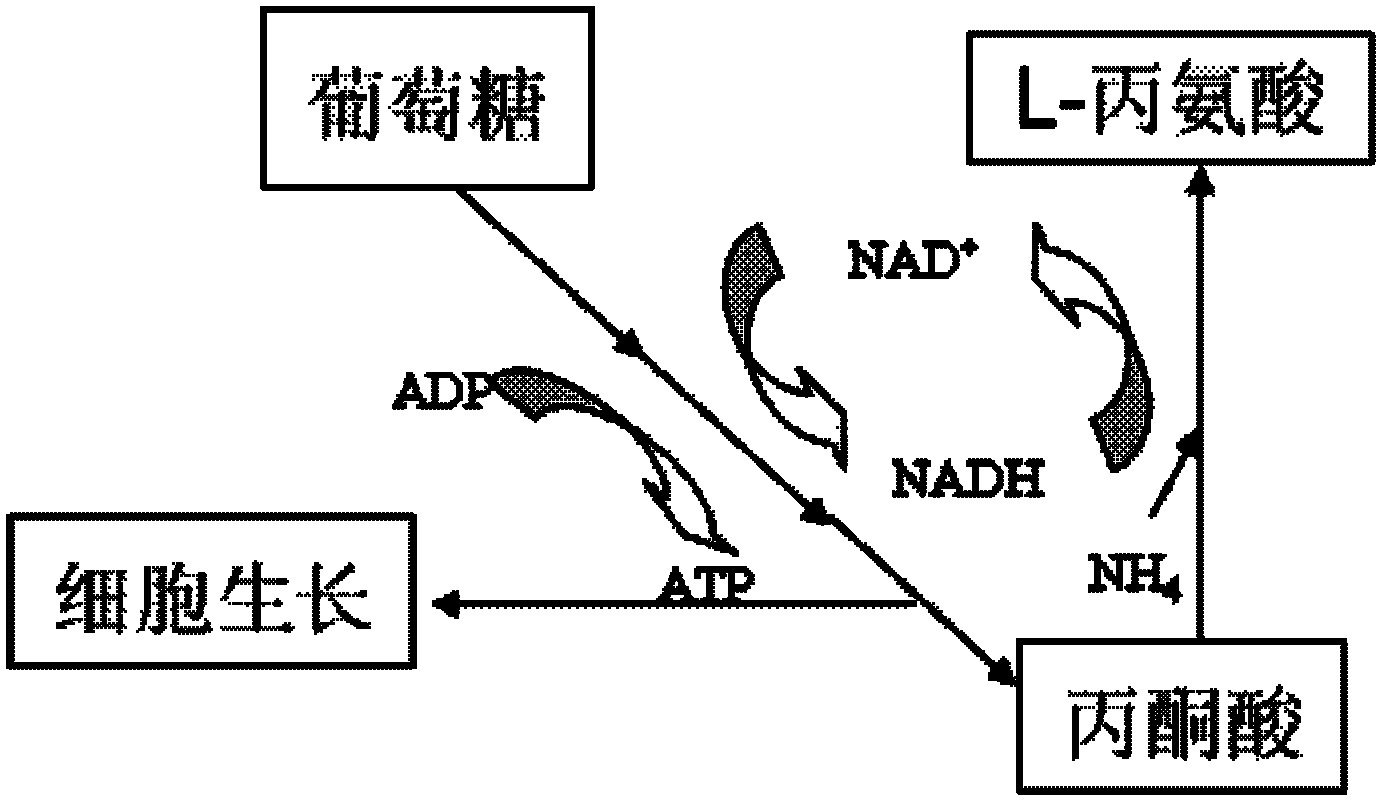
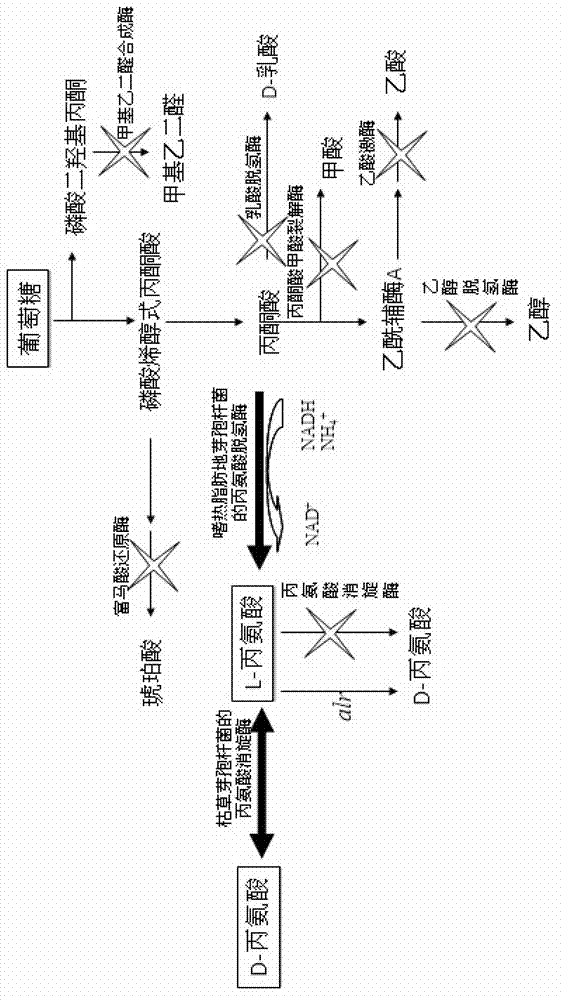
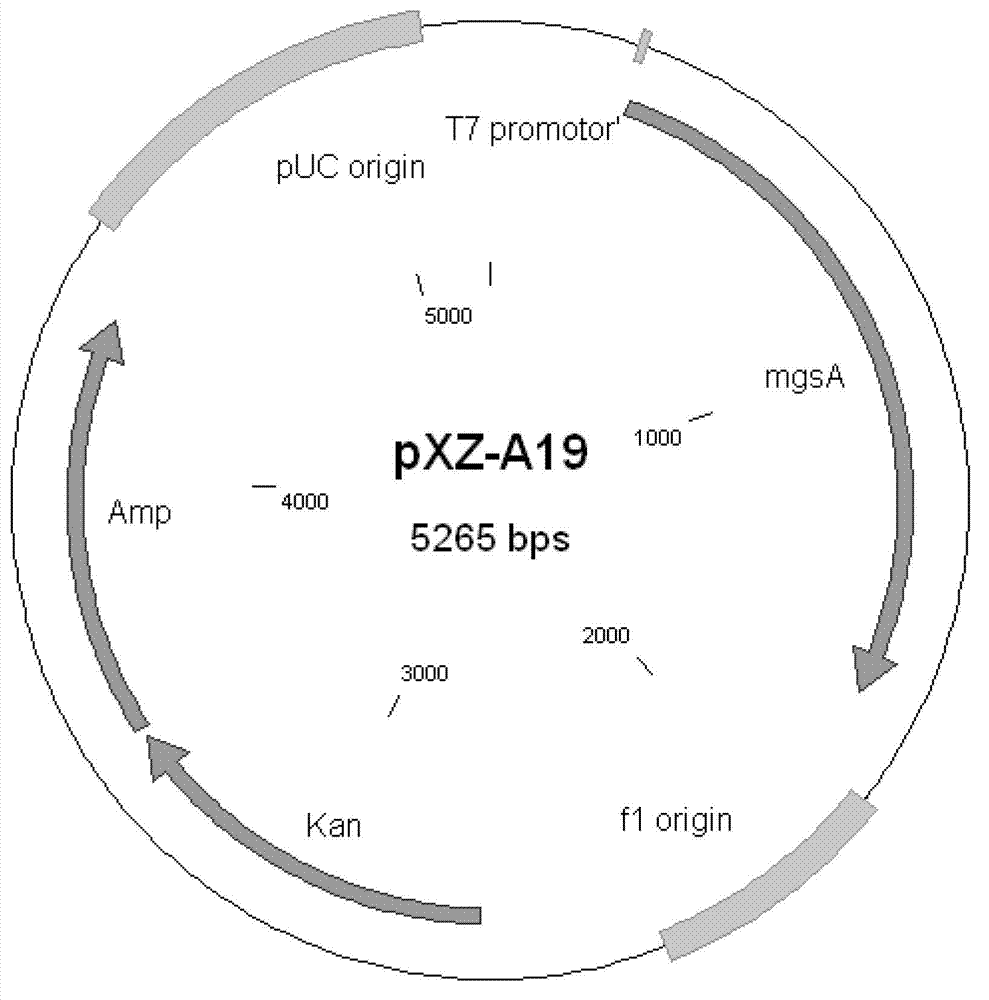
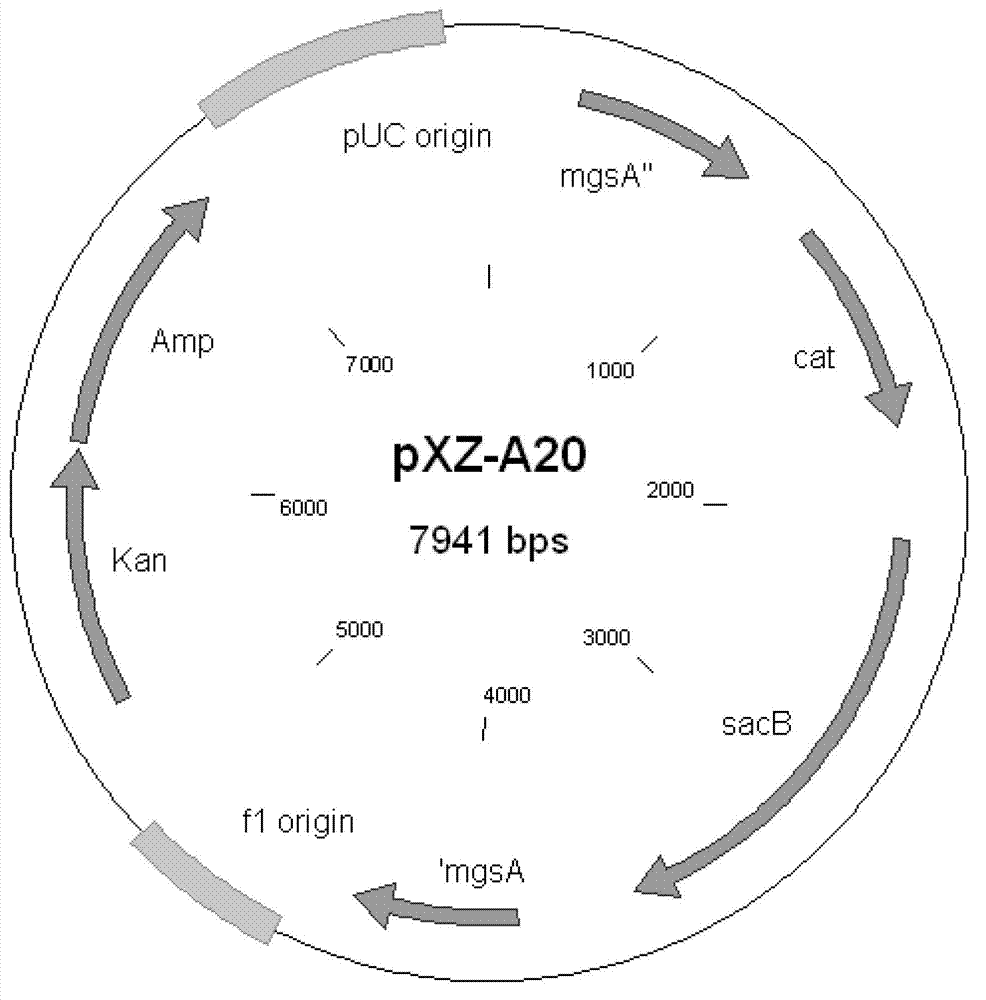
The invention discloses an XZ-A26 bacterial strain for producing L-alanine with high yield, wherein the preservation number is CGMCC No.4036. The XZ-A26 bacterial strain has the ability of fermenting to produce high-concentration L-alanine and is genetic engineering bacteria prepared by integrating the L-alanine dehydrogenase gene on Escherichia Coli chromosome of a lipase gene from Geobacillus Stearothermophilus at the lactic dehydrogenase of Escherichia coli ATCC 8739 chromosome, then sequentially removing a pyruvate formate-lyase gene, an ethanol dehydrogenase gene, an acetokinase gene, a fumarate reductase gene and an alanine racemase gene of the escherichia coli chromosome and then continuously subculturing in a fermentation tank. The invention also relates to a construction method of the bacterial strain and the application of the bacterial strain in preparing the L-alanine. According to the metabolic engineering method, the escherichia coli CGMCC No.4036 capable of fermenting to produce the high-concentration L-alanineis constructed, and the yield of the L-alanine produced by fermenting the bacterial strain is as high as 115g / L. The invention is suitable for the industrial production of L-alanine.
Owner:ANHUI HUAHENG BIOTECH
Engineering bacterium of fermenting production of optical pure L-lactate by utilizing xylose and construction thereof
InactiveCN102433293AHigh lactic acid productionPromote accumulationBacteriaMicroorganism based processesAcetic acidKanamycin
The invention discloses an engineering bacterium of fermentation production of optical pure L-lactate by utilizing xylose, as well as a construction method and an application thereof. The engineering bacterium is constructed in the following steps of: synthesizing a kar kanamycin resistant gene, and inserting the kar kanamycin resistant gene into a shuttle vector of escherichia coli and thermophilic anaerobic bacillus; amplifying a certain sequence of an acetokinase coding gene, which is a pta-up sequence; inserting the pta-up sequence into the shuttle vector of the escherichia coli and thermophilic anaerobic bacillus to construct a suicide vector 1; amplifying a certain sequence of a phosphotransacetylase coding gene, which is an ack-down sequence; inserting the ack-down sequence into the suicide vector 1 to obtain a suicide vector pPuKAd; converting the suicide vector pPuKAd into the thermophilic anaerobic bacillus, performing resistance screening to obtain the optical pure L-lactate by utilizing fermentation of xylose. The engineering bacterium of the invention is used for fermentation production of lactate, acetic acid is not generated, and a carbon metabolic flux is distributed once again, so that massive accumulation of the target product L-lactate is promoted, and the optical purity of the L-lactate is over 99.5 percent.
Owner:SOUTH CHINA UNIV OF TECH
Enzymatic synthesis method and application of 3',5'-adenosine diphosphate (PAP)
PendingCN110982864ABig cost advantageEasy to store and transportTransferasesFermentationEnzymatic synthesisPhosphoric acid
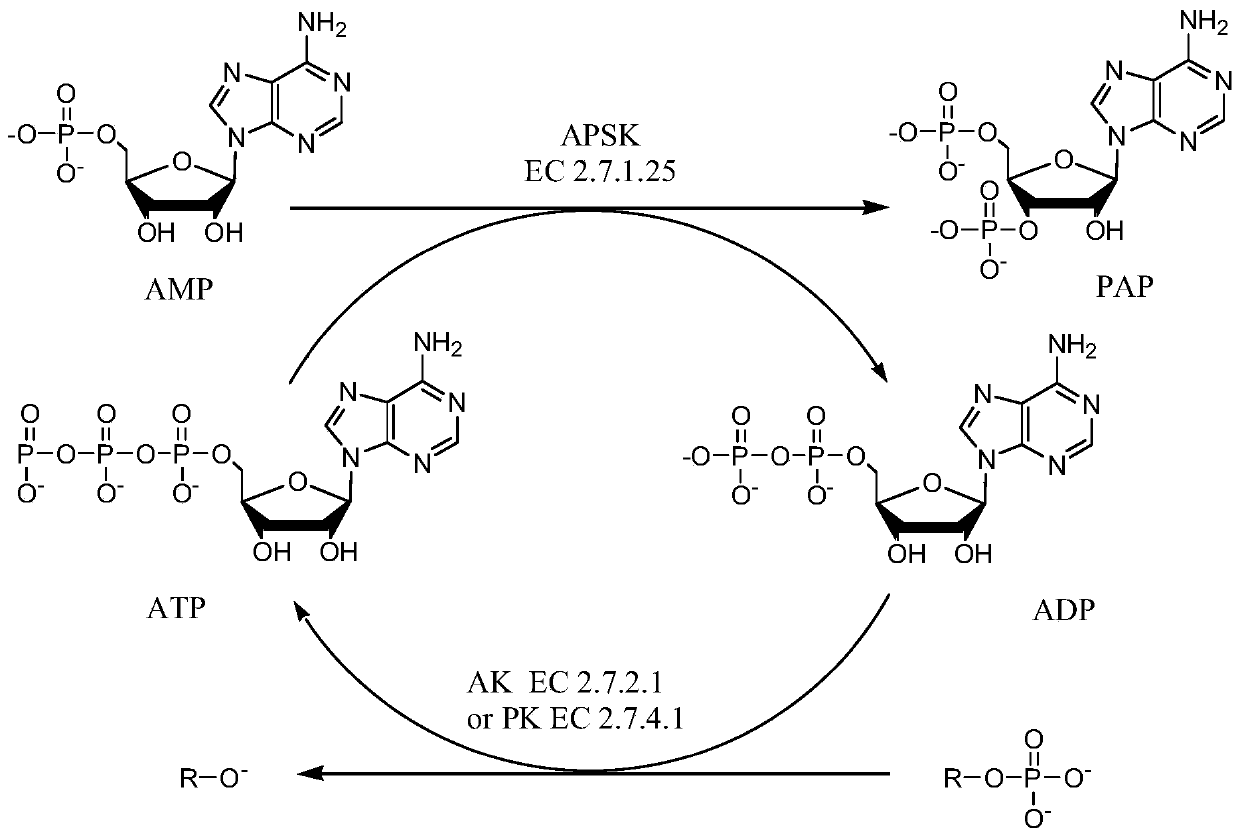
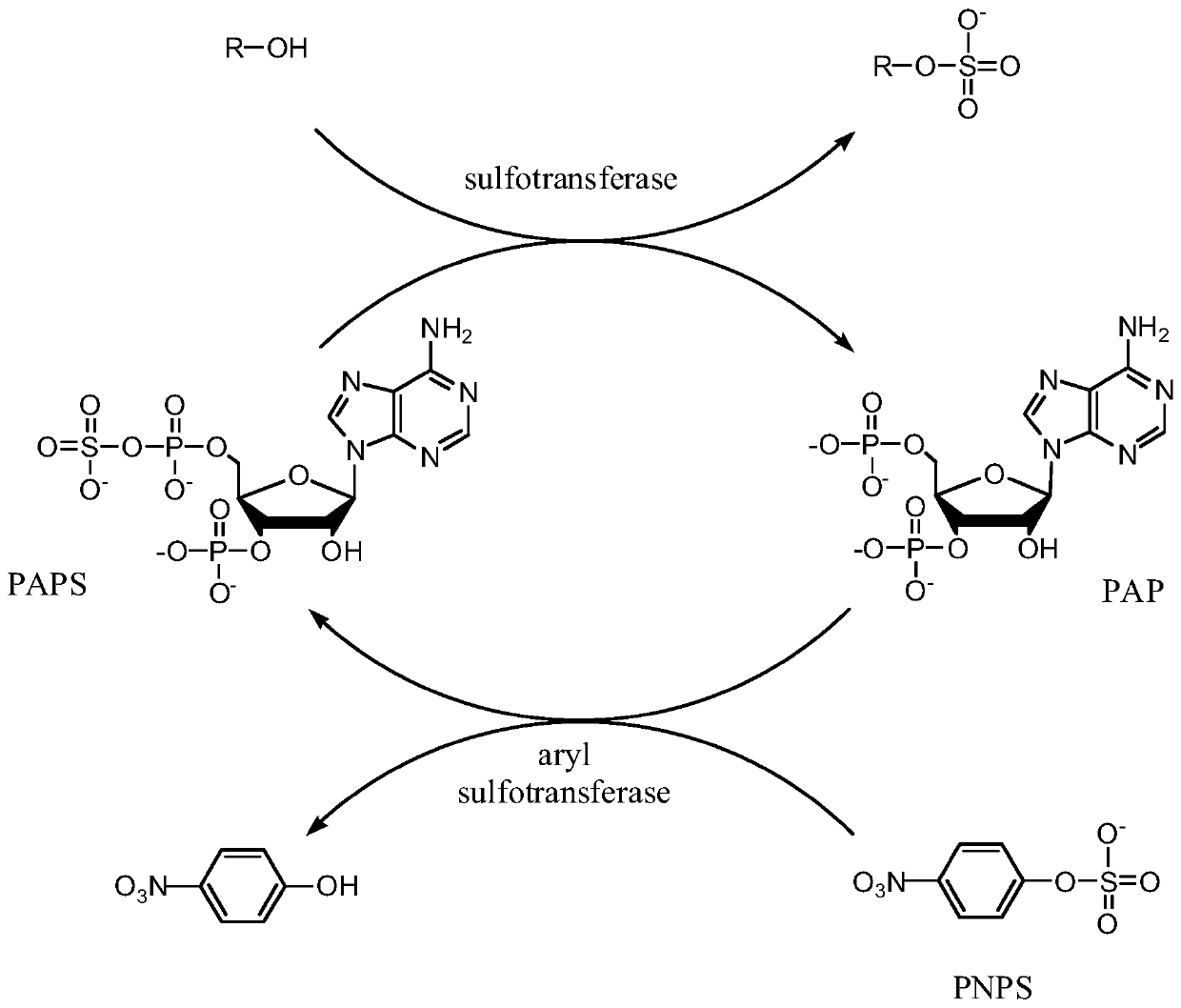
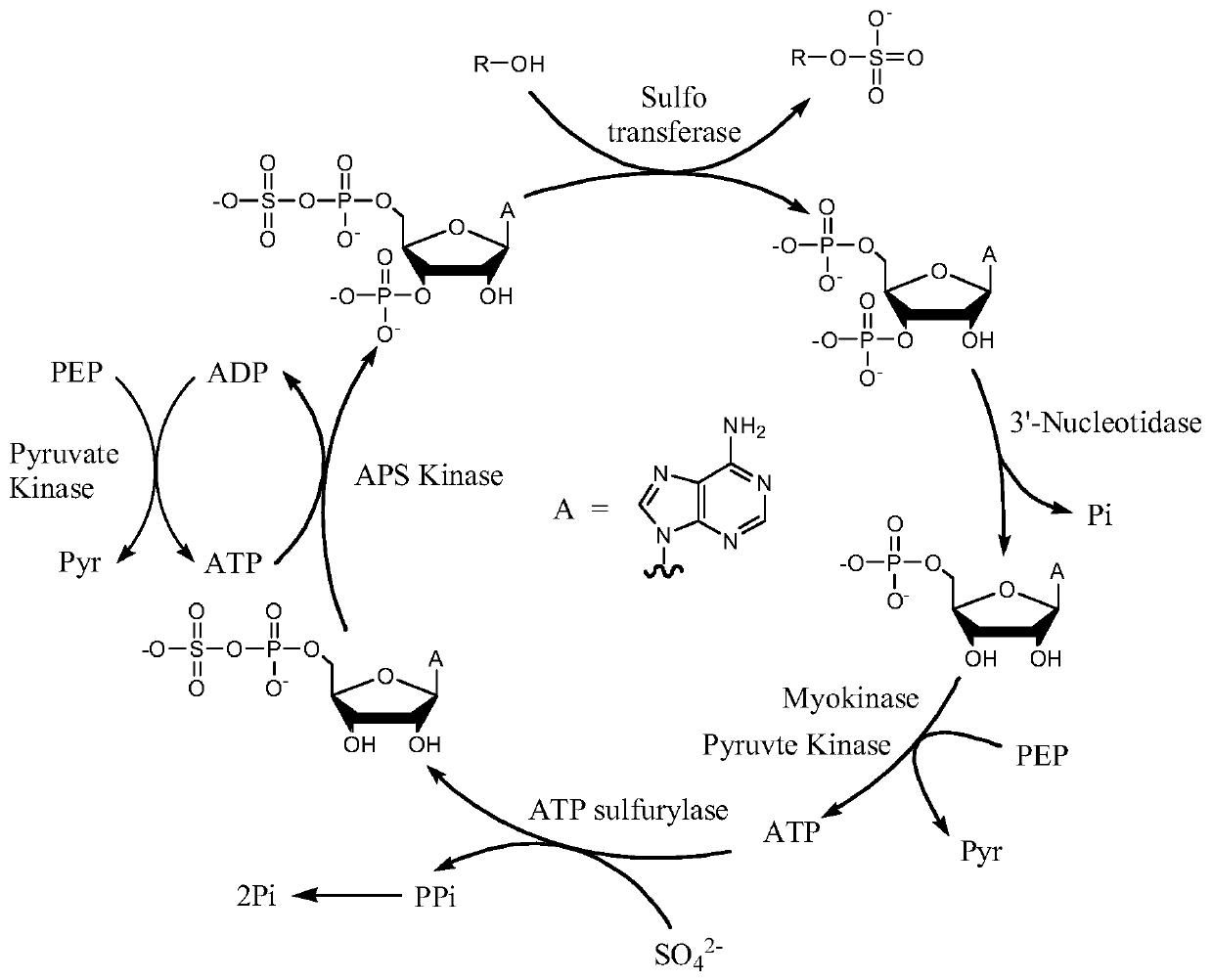
The invention provides an enzymatic synthesis method of PAP. The enzymatic synthesis method comprises the following steps: generating PAP with 5'-adenosine monophosphate (AMP) as a substrate, 5'-phosphosulfated kinase (APSK) as a catalyst and 5'-adenosine triphosphate (ATP) or 5'-adenosine diphosphate (ADP) as a coenzyme; and synthesizing ATP with acetylphosphoric acid or polyphosphoric acid as aphosphate group donor and acetokinase (AK) or polyphosphoric kinase (PK) as a catalyst, thereby realizing ATP circulation (as shown in Figure 1). The method has the advantages that the PAP is synthesized in one step by taking AMP as a substrate, and that the theoretical utilization rate of phosphorus reaches 100%.
Owner:孙军亭 +2
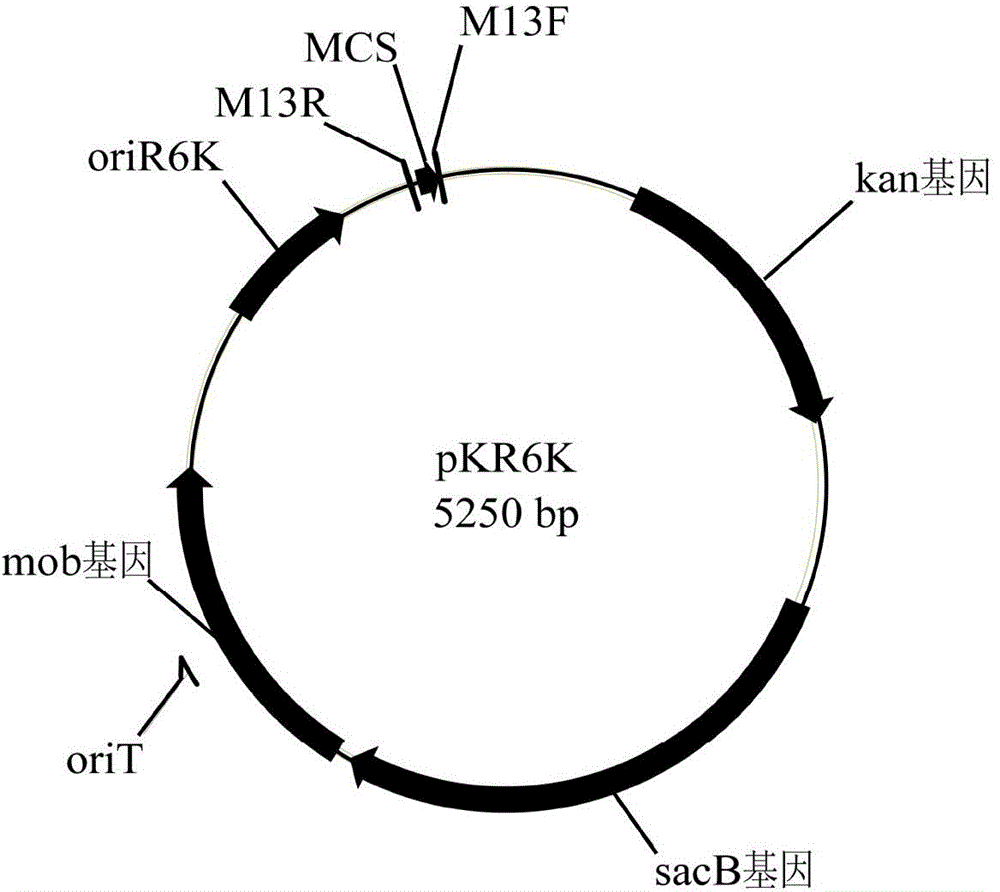
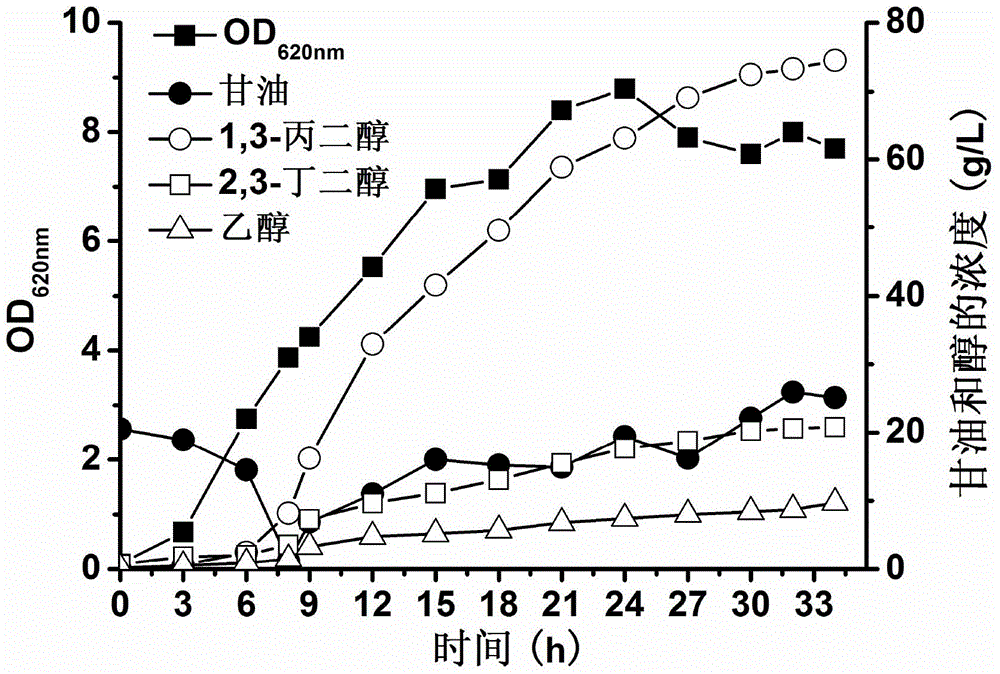
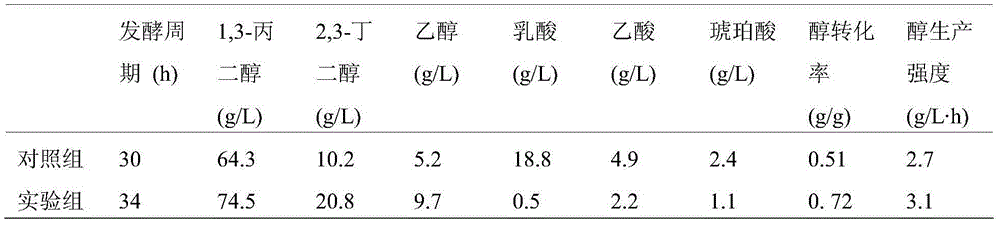
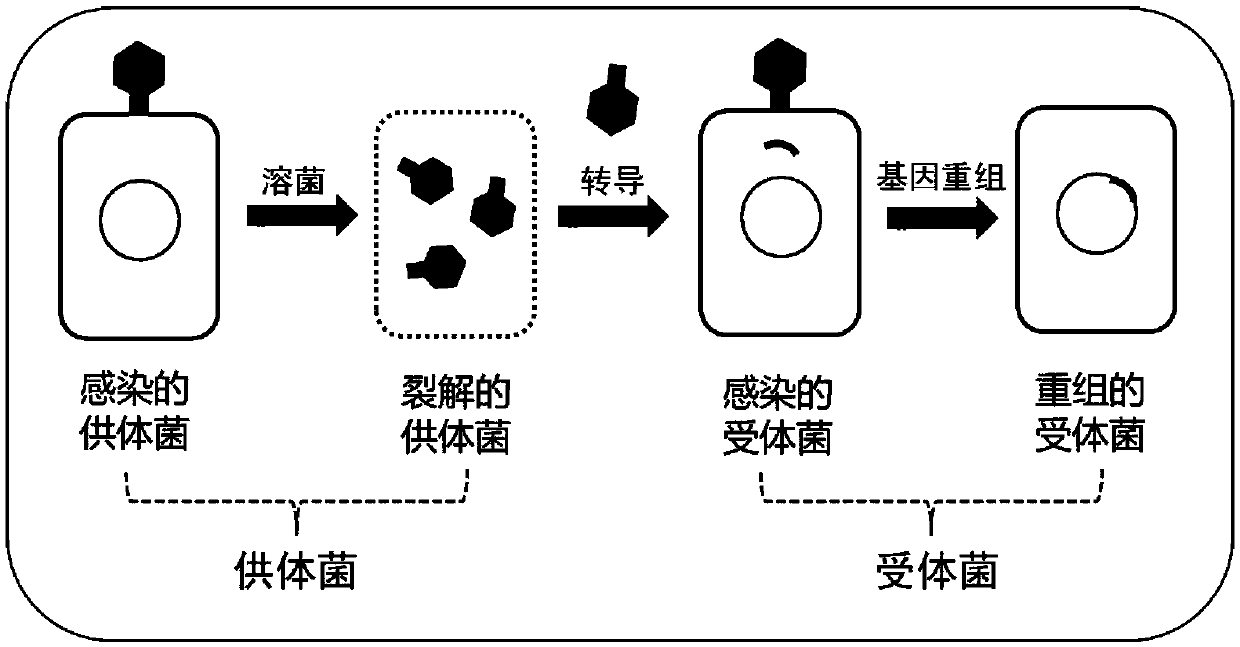
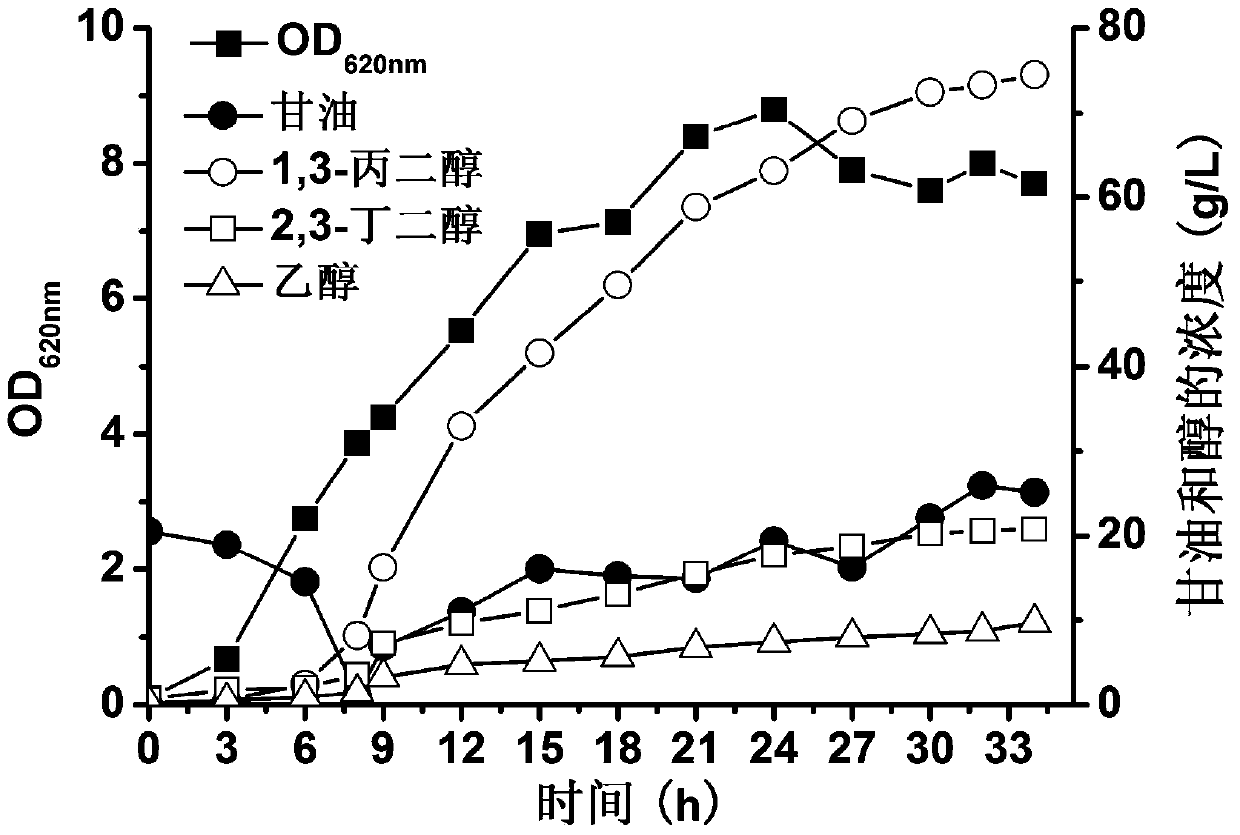
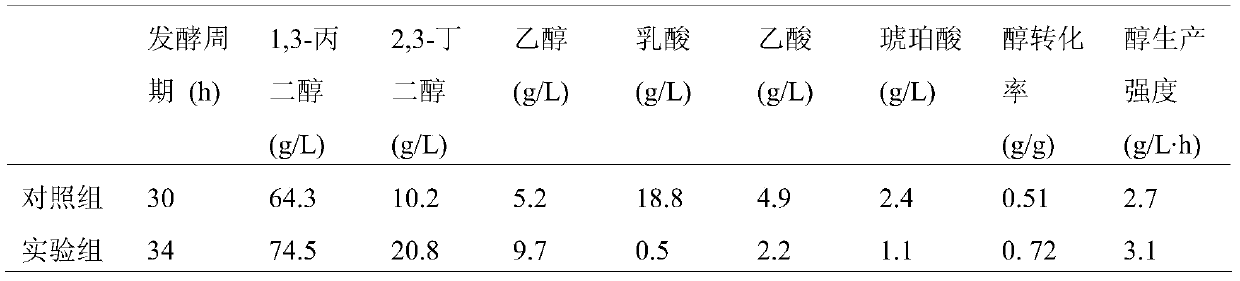
Engineered bacterium lacking organic acid production paths and use thereof in co-production of 1,3-propanediol, 2,3-butanediol and ethanol
ActiveCN106148255AReduce production processIncrease productionBacteriaBiofuels2,3-ButanediolMicrobial transformation
The invention discloses an engineering bacterium lacking organic acid production paths and use thereof in co-production of 1,3-propanediol, 2,3-butanediol and ethanol. Lactic dehydrogenase gene of lactic acid synthesis path of Klebsiella, acetate kinase gene and pyruvic oxidase gene in acetic acid synthesis path, and fumarate reductase gene and isocitrate lyase gene of succinic acid synthesis path are inactivated, so that production of organic acids in metabolism of glycerol of the Klebsiella can be reduced, and yield and conversion rate of alcohols such as1,3-propanediol, 2,3-butanediol and ethanol can be improved, and the co-production of the 1,3-propanediol, 2,3-butanediol and ethanol can be achieved. As a significant reduction in the production of the organic acids, the extraction process of the alcohols can be simplified. The production efficiency of production of high value products by microbial conversion of glycerol can be improved, the production cost is reduced, and the engineering bacterium has important application value.
Owner:ZHANGJIAGANG GLORY CHEM IND CO LTD
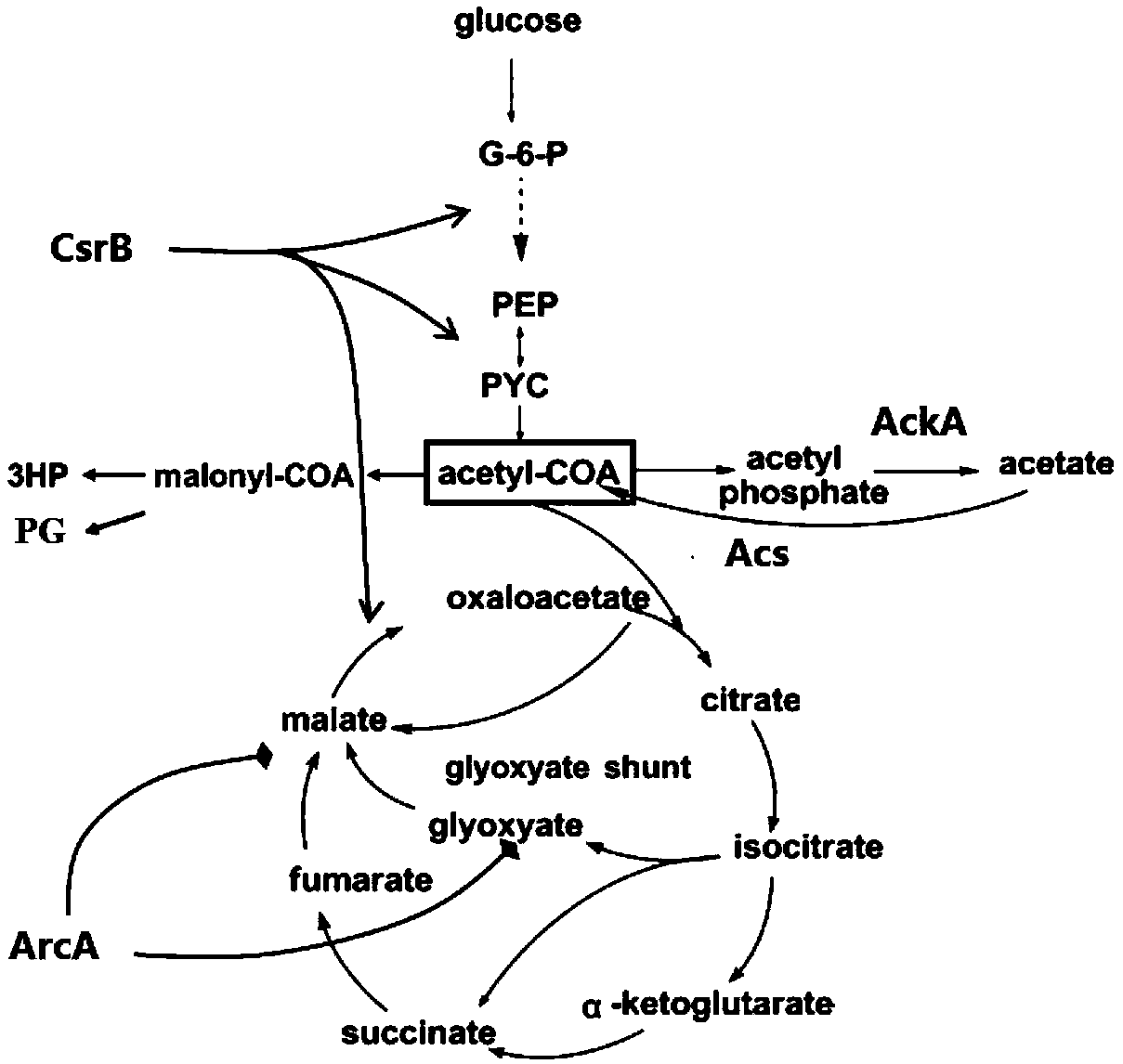
General chassis cell for synthesizing acetylcoenzyme A derived product and construction method and application of general chassis cell
ActiveCN110656075AVersatilityBiosynthesis achievedBacteriaMicroorganism based processesBiotechnologyEnzyme Gene
The invention discloses a general chassis cell for synthesizing an acetylcoenzyme A derived product and a construction method and application of the general chassis cell, and belongs to the technicalfield of genetic engineering. The chassis cell adopts escherichia coli as a start bacterial strain, overall situation regulating factors arcA and acetate kinase genes ackA in escherichia coli genome are knocked out, and carbon storage regulatory factors csrB and acetylcoenzyme A synthetase genes acs are subjected to over expression. Besides, the invention further provides a preparation method of the chassis cell, and a method for producing the acetylcoenzyme A derived product by using the chassis cell. The invention firstly completes establishment of the general chassis cell through biosynthesis of the acetylcoenzyme A derived product, the purpose of reforming production is realized, and the method has great innovativeness and application potential. The chassis cell is suitable for biosynthesis of the acetylcoenzyme A derived product.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Homo-succinic acid producing microorganism variant and process for preparing succinic acid using the same
ActiveUS20100159544A1High succinic acid productivityHigh yieldBacteriaHybrid cell preparationMicroorganismLactate dehydrogenase
The present invention relates to microbial variants producing homo-succinic acid at high yields and a method for producing homo-succinic acid using the same, more particularly, to a microbial variant constructed by disrupting a lactate dehydro-genase-encoding gene (idhA) and an acetate kinase-encoding gene (ackA), as well as a method for producing homo-succinic acid at high concentration, which comprises culturing such variants using glucose as a carbon source in anaerobic conditions.
Owner:KOREA ADVANCED INST OF SCI & TECH
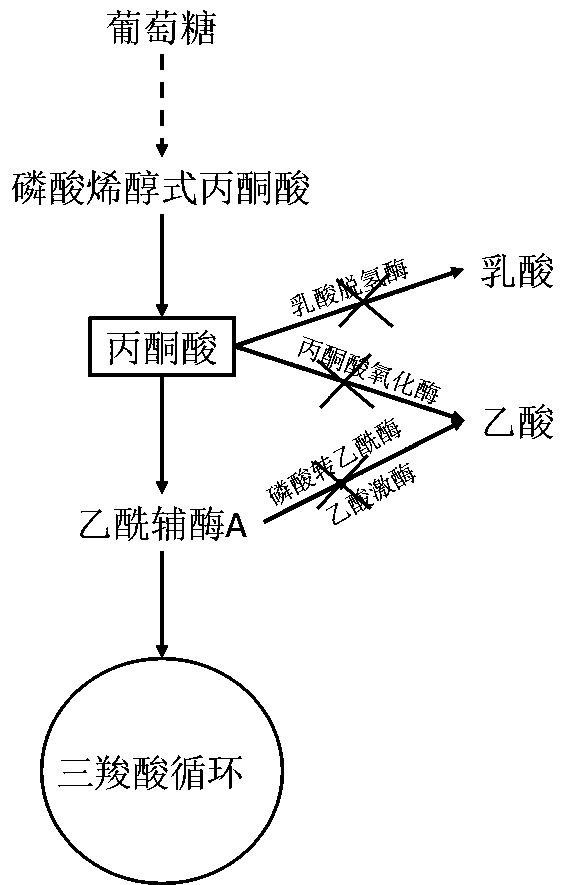
Method for increasing pyruvic acid accumulated in escherichia coli
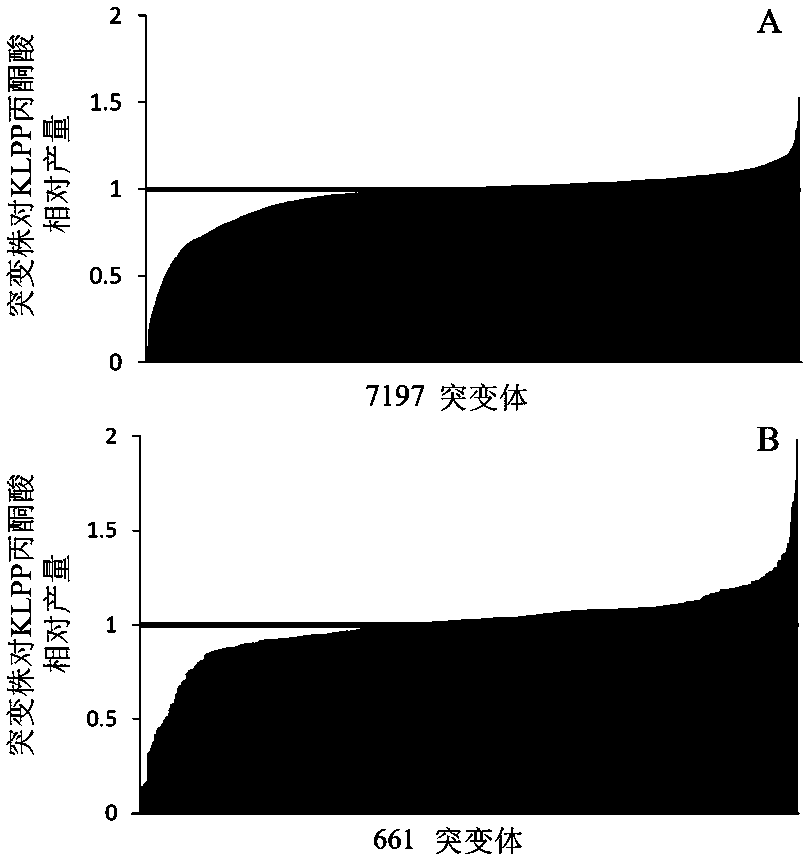
The invention belongs to the technical field of microorganism genetic engineering and in particular relates to an escherichia coli genetically engineered bacterium establishment method for increasingaccumulation of pyruvic acid, and production and application. The genetically engineered bacterium provided by the invention is established by using a method of the following steps: deleting encodinggenes of lactic dehydrogenase, encoding genes of pyruvic oxidase, encoding genes of phosphotransacetylase and encoding genes of acetokinase from wild derived escherichia coli, and establishing an escherichia coli genetically engineered bacterium KLPP with accumulated pyruvic acid. From KLPP, a mutation library is established through Tn5 transposons, a mutation strain with increased pyruvic acid accumulation is screened in a high flux manner, and genes for increasing pyruvic acid accumulation can be found through whole genome sequencing.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Construction method of bacillus subtilis engineering bacteria for high yielding of alanine
InactiveCN104593405AIncrease productionBacteriaMicroorganism based processesL-Lactate dehydrogenaseGene mutation
The invention discloses a preparation method of bacillus subtilis engineering bacteria for high yielding of alanine, wherein the method comprises that alanine-synthesized branched metabolism genes L-lactate dehydrogenase gene ldh and acetate kinase gene ackA on a B.subtilis 168 chromosome are knocked out by using a homologous recombination method, and a mutant strain is obtained. The single-gene mutant strain has the preservation number of IBL23-ldh, the construction of the mutant strain is achieved by knocking out a part of the lactate dehydrogenase gene of bacillus subtilis; a double-gene mutant strain is obtained by again knocking out the acetate kinase gene based on the single-gene mutant strain and has the number for IBL23-ldh / ackA.
Owner:ANHUI UNIVERSITY
A kind of preparation method of glutamic acid dipeptide
ActiveCN106834394BAchieve recyclingWide variety of sourcesTransferasesPeptidesDipeptidePhosphorylation
Owner:TIANJIN UNIV OF SCI & TECH
Ammonia ion diagnosis/measuring reagent kit and ammonia ion concentration determination method
InactiveCN101464346AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryPyrophosphate
The invention relates to a kit for diagnosing / measuring ammonia (ions) by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of ammonia (ions), and belongs to the technical field of medical / food / environmental inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, glutamic acid, adenyl pyrophosphate, acetyl phosphate, glutamine synthetase, acetokinase, aldehyde dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree / velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the concentration of ammonia (ions).
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Engineered microorganism producing homo-succinic acid and method for preparing succinic acid using the same
ActiveUS9217138B2Increase productivityHigh rateBacteriaStable introduction of DNAHigh concentrationMannheimia
The present invention relates to a mutant microorganism, which is selected from the group consisting of genus Mannheimia, genus Actinobacillus and genus Anaerobiospirillum, producing homo-succinic acid and a method for producing homo-succinic acid using the same, and more particularly to a mutant microorganism producing succinic acid at a high concentration while producing little or no other organic acids in anaerobic conditions, which is obtained by disrupting a gene encoding lactate dehydrogenase (ldhA), a gene encoding phosphotransacetylase (pta), and a gene encoding acetate kinase (ackA), without disrupting a gene encoding pyruvate formate lyase (pfl), as well as a method for producing succinic acid using the same. The inventive mutant microorganism has the property of having a high growth rate and succinic acid productivity while producing little or no organic acids, as compared to the prior strains producing succinic acid. Thus, the inventive mutant microorganism is useful to produce succinic acid for industrial use.
Owner:KOREA ADVANCED INST OF SCI & TECH
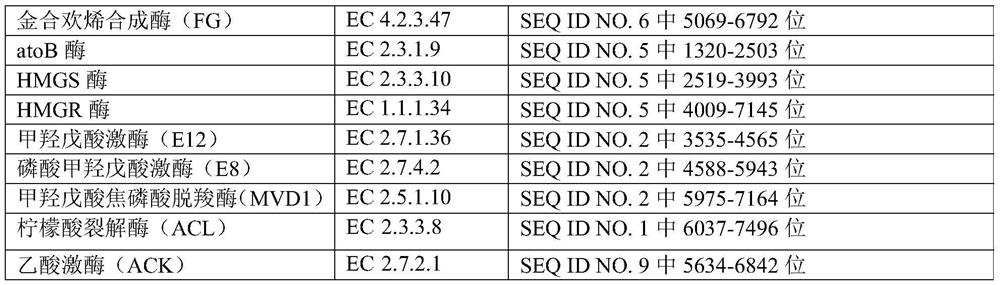
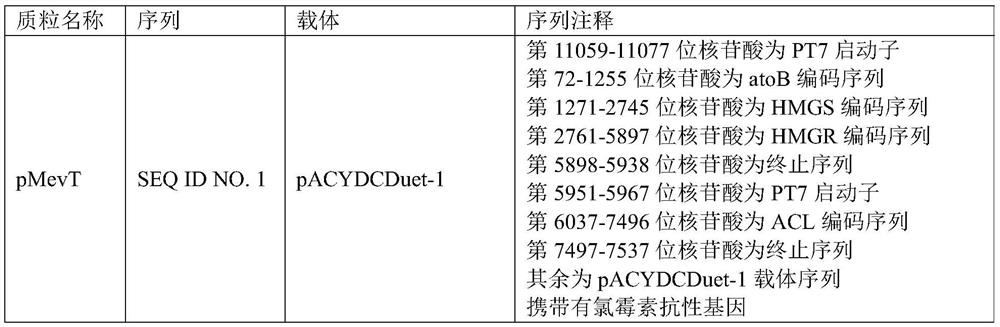
Method for preparing farnesene by cellulose, and engineering strain
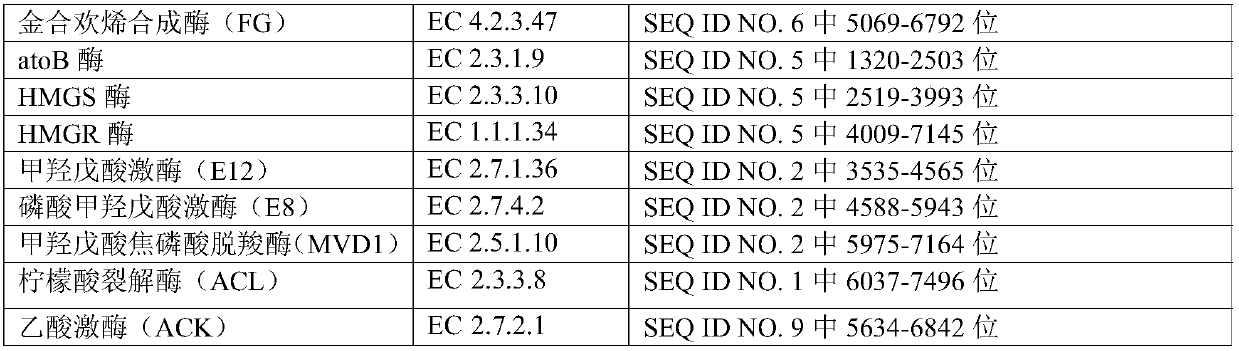
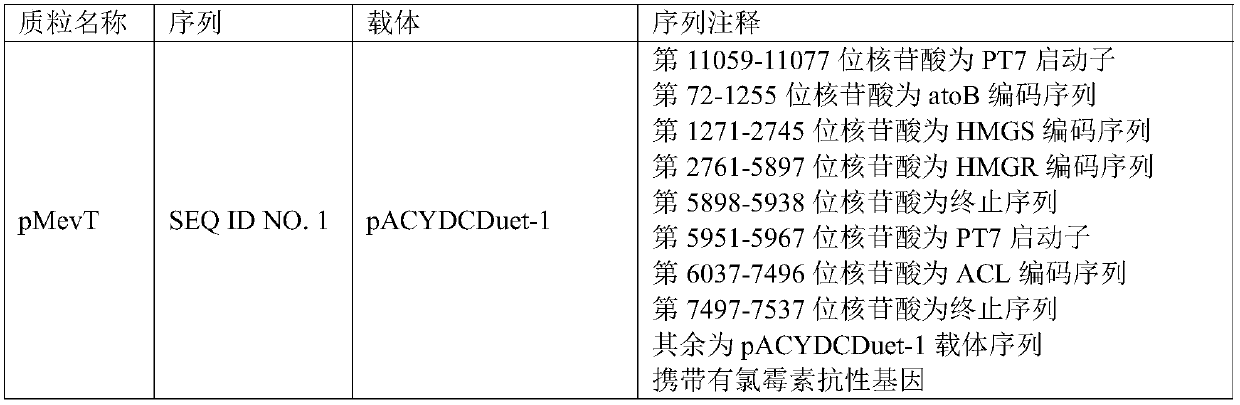
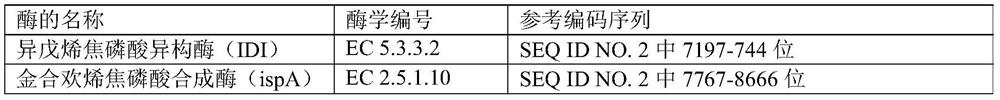
The invention provides an engineering strain, which is escherichia coli of which the collection number is CGMCC (China General Microbiological Culture Collection Center) NO. 14821. The invention alsoprovides a method for preparing engineering bacteria. The method comprises the following steps of: after acetokinase is knocked into the genome of the host bacteria of the engineering bacteria, transferring in a recombinant plasmid, and enabling the transferred host bacteria of the engineering bacteria to express the acetokinase and express a gene inserted into the recombinant plasmid, wherein therecombinant plasmid comprises a plasmid disclosed in SEQ ID NO.1, a plasmid disclosed in SEQ ID NO.2 and a plasmid disclosed in SEQ ID NO.7. The invention also discloses the engineering bacteria prepared by the method, the above engineering strain, a purpose of the engineering bacteria, and a method for preparing farnesene by cellulose. Through the above technical scheme, a yield for producing the farnesene by the fermentation of cellulose hydrolysate can be obviously improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
An engineering strain and method for preparing farnesene from cellulose
The present disclosure provides an engineering strain, which is Escherichia coli with a preservation number of CGMCC NO.14821. The present disclosure also provides a method for preparing an engineering bacterium, the method comprising: knocking in acetate kinase in the genome of the engineering bacterium host bacterium and then transferring the recombinant plasmid, so that the engineered bacterium host bacterium after the transfer can pass through expresses acetate kinase and can express the gene inserted in the recombinant plasmid; the recombinant plasmid includes the plasmid shown in SEQ ID NO.1, the plasmid shown in SEQ ID NO.2 and the plasmid shown in SEQ ID NO.7 plasmids indicated. The present disclosure also provides the engineering bacteria prepared by the method, the engineering strain and the application of the engineering bacteria, and a method for preparing farnesene from cellulose. The present disclosure significantly improves the yield of farnesene produced by fermenting cellulose hydrolyzate through the above technical scheme.
Owner:CHINA PETROLEUM & CHEM CORP +1
Engineering bacteria with deletion of organic acid production pathway and its application in co-production of 1,3-propanediol, 2,3-butanediol and ethanol
ActiveCN106148255BReduce production processIncrease productionBacteriaBiofuels2,3-ButanediolMicrobial transformation
The invention discloses an engineering bacterium lacking organic acid production paths and use thereof in co-production of 1,3-propanediol, 2,3-butanediol and ethanol. Lactic dehydrogenase gene of lactic acid synthesis path of Klebsiella, acetate kinase gene and pyruvic oxidase gene in acetic acid synthesis path, and fumarate reductase gene and isocitrate lyase gene of succinic acid synthesis path are inactivated, so that production of organic acids in metabolism of glycerol of the Klebsiella can be reduced, and yield and conversion rate of alcohols such as1,3-propanediol, 2,3-butanediol and ethanol can be improved, and the co-production of the 1,3-propanediol, 2,3-butanediol and ethanol can be achieved. As a significant reduction in the production of the organic acids, the extraction process of the alcohols can be simplified. The production efficiency of production of high value products by microbial conversion of glycerol can be improved, the production cost is reduced, and the engineering bacterium has important application value.
Owner:ZHANGJIAGANG GLORY CHEM IND CO LTD
A recombinant expression vector, recombinant expression host and method for synthesizing adenosine triphosphate
ActiveCN108753808BImprove conversion rateHigh yieldFermentationVector-based foreign material introductionAdenylate kinaseAdenosine
The invention belongs to the field of bioengineering and relates to the preparation of adenosine triphosphate. The invention provides a recombinant expression vector and a recombinant expression host comprising the vector, the recombinant expression vector comprises polynucleotides encoding adenosine kinase, adenylate kinase and acetate kinase; the invention also provides the use of the A method for the synthesis of adenosine triphosphate by a recombinant expression host. The invention improves the adenosine conversion rate, ATP production rate and reaction efficiency by using the whole cell of the recombinant expression host to catalyze the synthesis of adenosine triphosphate, and meanwhile, the components of the reaction system are simple and the cost is low.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Application of protein of high-pathogenicity staphylococcus hyicus to subunit vaccine
InactiveCN106474465AImprove the immunityAvoid infectionAntibacterial agentsAntibody medical ingredientsHighly pathogenicSecretory protein
The invention discloses application of protein of high-pathogenicity staphylococcus hyicus to a subunit vaccine. The secreted protein has a relatively good immune protection capability and can resist infection on mice by the staphylococcus hyicus. The amino acid sequence of the protein of the staphylococcus hyicus is shown as SEQ ID NO: 1. The protein acetate kinase of the staphylococcus hyicus (S.hyicus, named as ZC-4) has relatively good immunogenicity; prokaryotic expression recombinant protein and pig immune blood serum of the ZC-4 have a mutual effect, and specificity with a relatively high level can be induced. A toxin attacking protection efficacy experiment shows that the resistance on the staphylococcus hyicus of the mice and piglets can be improved through the protein, and the infection of the staphylococcus hyicus can be effectively prevented through taking the protein as the subunit vaccine.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com