Recombinant expression vector, recombinant expression host and method for synthesizing ATP (adenosine triphosphoric acid) from recombinant expression host
An expression vector and adenylate kinase technology, applied in the field of preparation of adenosine triphosphate, can solve the problems of large product batch quality differences, difficult control of the reaction process, difficult separation and purification, etc., to achieve industrial production and easy subsequent purification Industry, the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
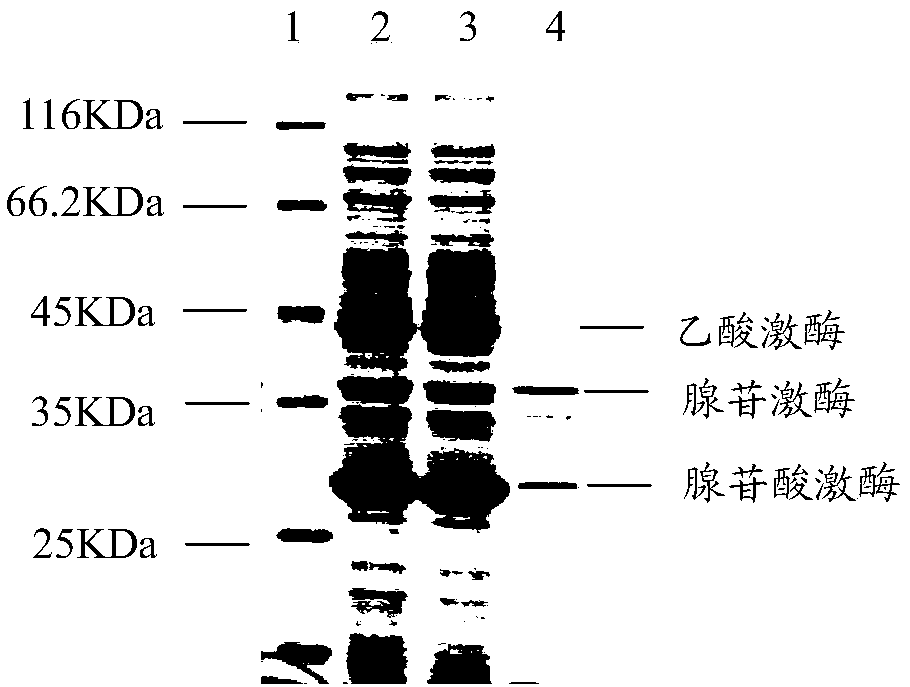
[0034] The construction of embodiment 1 recombinant escherichia coli
[0035] Experimental materials and reagents:
[0036] Escherichia coli BL21(DE3) (E.coil BL21(DE3)): purchased from Invitrogen;
[0037] Escherichia coli DH5α (E.coil DH5α): purchased from Invitrogen;
[0038] Saccharomyces cerevisiae strain: China Center for Type Culture Collection, No. CCTCC AY 93175;
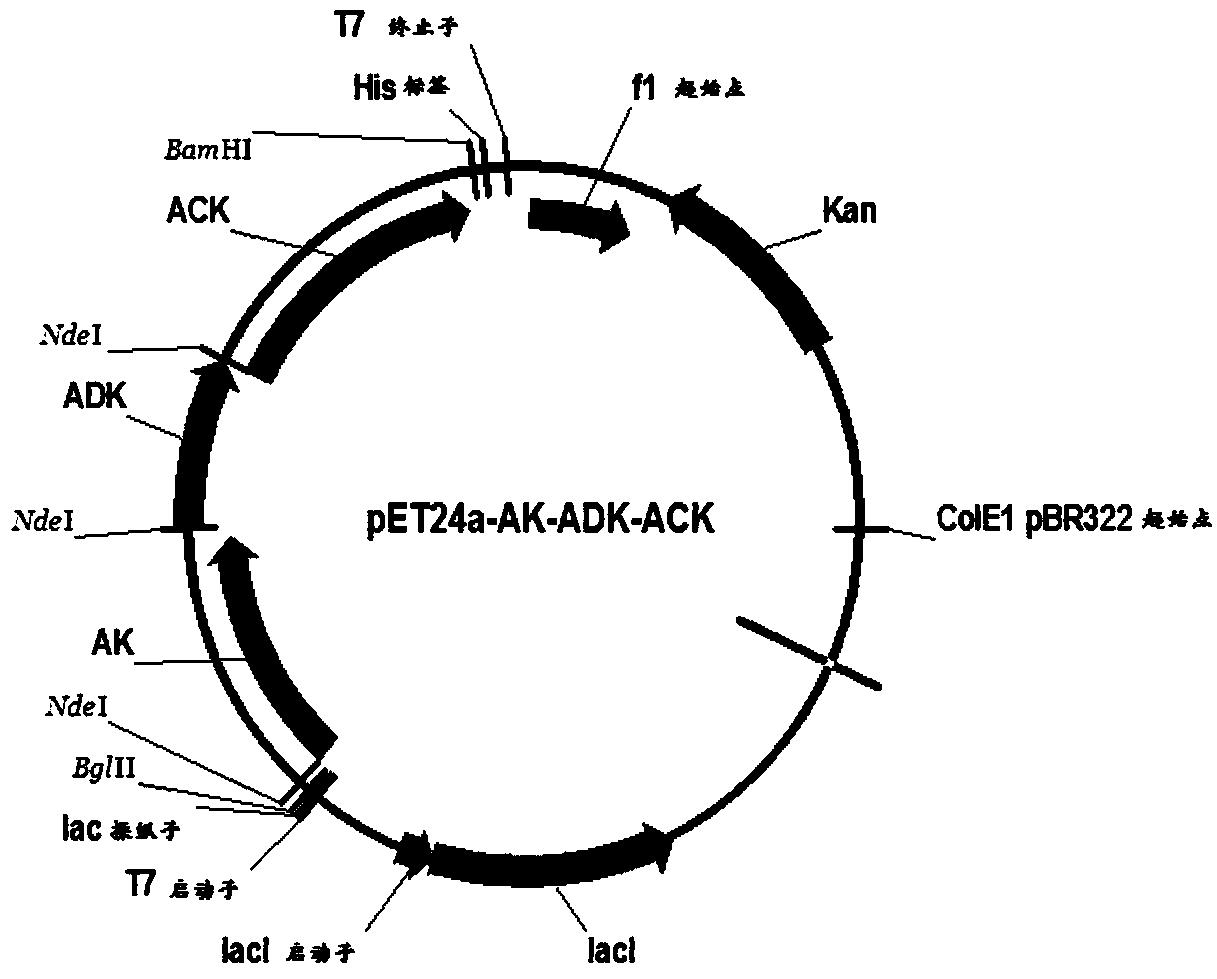
[0039] pET24a plasmid: used for expression plasmid, with T7 promoter, kanamycin resistance gene, purchased from NOVAGEN Company, Cat. No. 69749;
[0040] PCR primers for adenosine kinase, adenylate kinase and acetate kinase: synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.;
[0041] Pyrobest enzyme and 10×Pyrobest buffer: purchased from TaKaRa, product number R005;
[0042] Restriction endonuclease Nde I: purchased from TaKaRa, Cat. No. 1161A;
[0043] Restriction endonuclease BamH I: purchased from TaKaRa, Cat. No. 1010A;
[0044] Restriction endonuclease BglⅡ: purchased from TaKaRa, Cat. No. ...
Embodiment 2
[0077] Example 2 Recombinant Escherichia coli Whole Cell Catalytic Synthesis of ATP
[0078] In the reaction system (1L), add 10mmol / L adenosine, 50mmol / L borax, 50mmol / L ACP and 5mmol / L magnesium sulfate, add 10g / L thalline as above collected in embodiment 1 as enzyme source, adjust reaction The pH of the solution was 7.5, and the temperature was controlled at 35° C. during the reaction, and the reaction was carried out at a stirring speed of 150 r / min for 3 hours under mechanical stirring.
[0079] Collect the supernatant of the reaction solution, use high performance liquid chromatography (Agilent 1260) to analyze the concentration of residual adenosine by high performance liquid chromatography (HPLC) after filter sterilization; Diluted 10-fold, filter-sterilized and analyzed by HPLC.
[0080] HPLC conditions: Welch Ultimate XB-C18 chromatographic column (250mm×4.6mm I.D., 5μm particle size, purchased from Yuexu Technology (Shanghai) Co., Ltd., article number 00201-31043);...
Embodiment 3
[0083] Example 3 Recombinant Escherichia coli Whole Cell Catalytic Synthesis of ATP
[0084] Add 30mmol / L adenosine, 50mmol / L borax, 180mmol / L ACP and 10mmol / L magnesium sulfate in reaction system (1L), add 5g / L bacterium as enzyme source, adjust reaction solution pH to 7.5, during the reaction The temperature was controlled at 30° C., and the reaction was performed at a stirring speed of 150 r / min for 5 hours under mechanical stirring. ATP and adenosine concentrations were measured as described in Example 2. The generated ATP concentration was 15.1 g / L, and the conversion rate of adenosine was over 99% as calculated by the method described in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com