Use of creatine analogues and creatine kinase modulators for the prevention and treatment of glucose metabolic disorders
a technology of creatine kinase and analogues, which is applied in the field of use of creatine analogues and creatine kinase modulators for the prevention and treatment of glucose metabolic disorders, can solve the problems of significant drop in glucose levels in subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Creatine Compounds on Glucose Levels in Rats Bearing Tumors
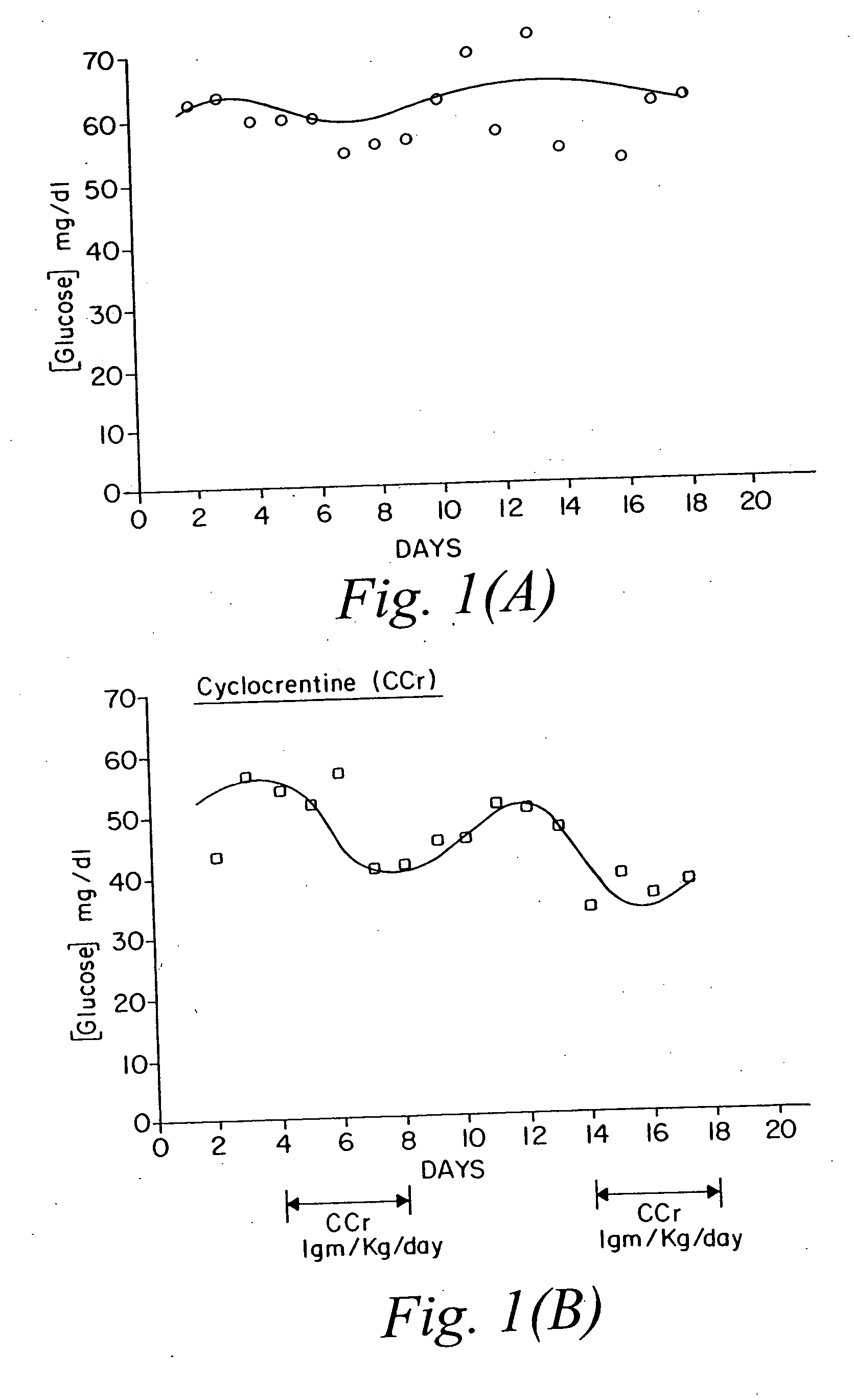
[0071] Two creatine compounds, creatine phosphate and cyclocreatine, were injected intravenously into tumor bearing rats, and the level of glucose in the rats was monitored. Beta guanidino propionic acid, also was administered. This compound was previously shown to have no effect on glucose levels in normal animals but was shown to modify glucose levels in NIDDM models. There was no specific reason for using tumor bearing rats, except convenience because the antitumor activity of these compounds also was being studied. The presence of the tumors should not have any effect on the ability of these compounds to regulate glucose levels.
[0072] The rats carrying the tumors were described by us previously (see, Teisher et al., Cancer Chemother. Pharmacol, 35: 411-416, 1995). The schedule and dose selected in these experiments was based on prior experience working with this class of compounds as anticancer or antiviral c...
example 2
Effect of Creatine Compounds on Glucose Levels in Rats Bearing Tumors
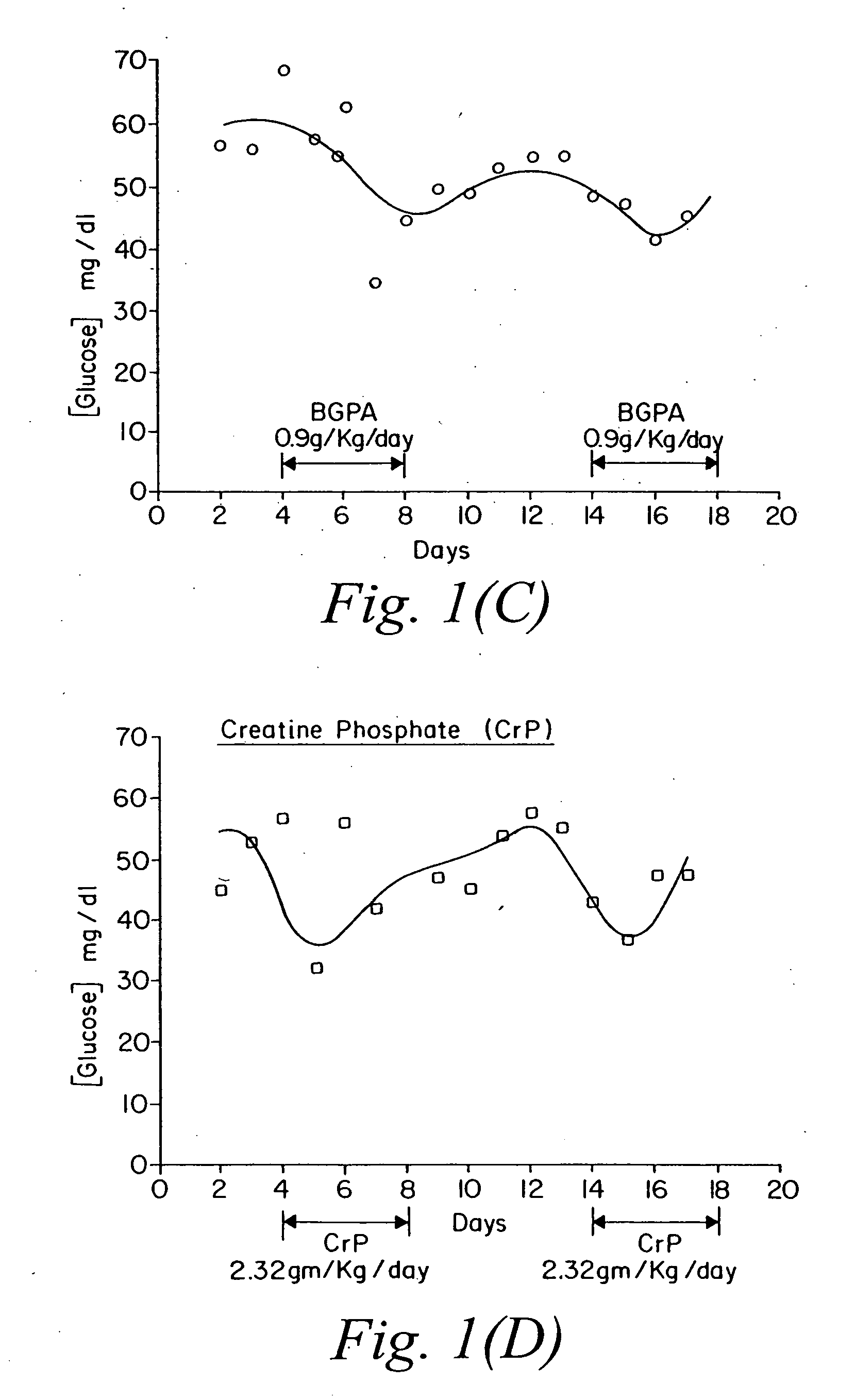
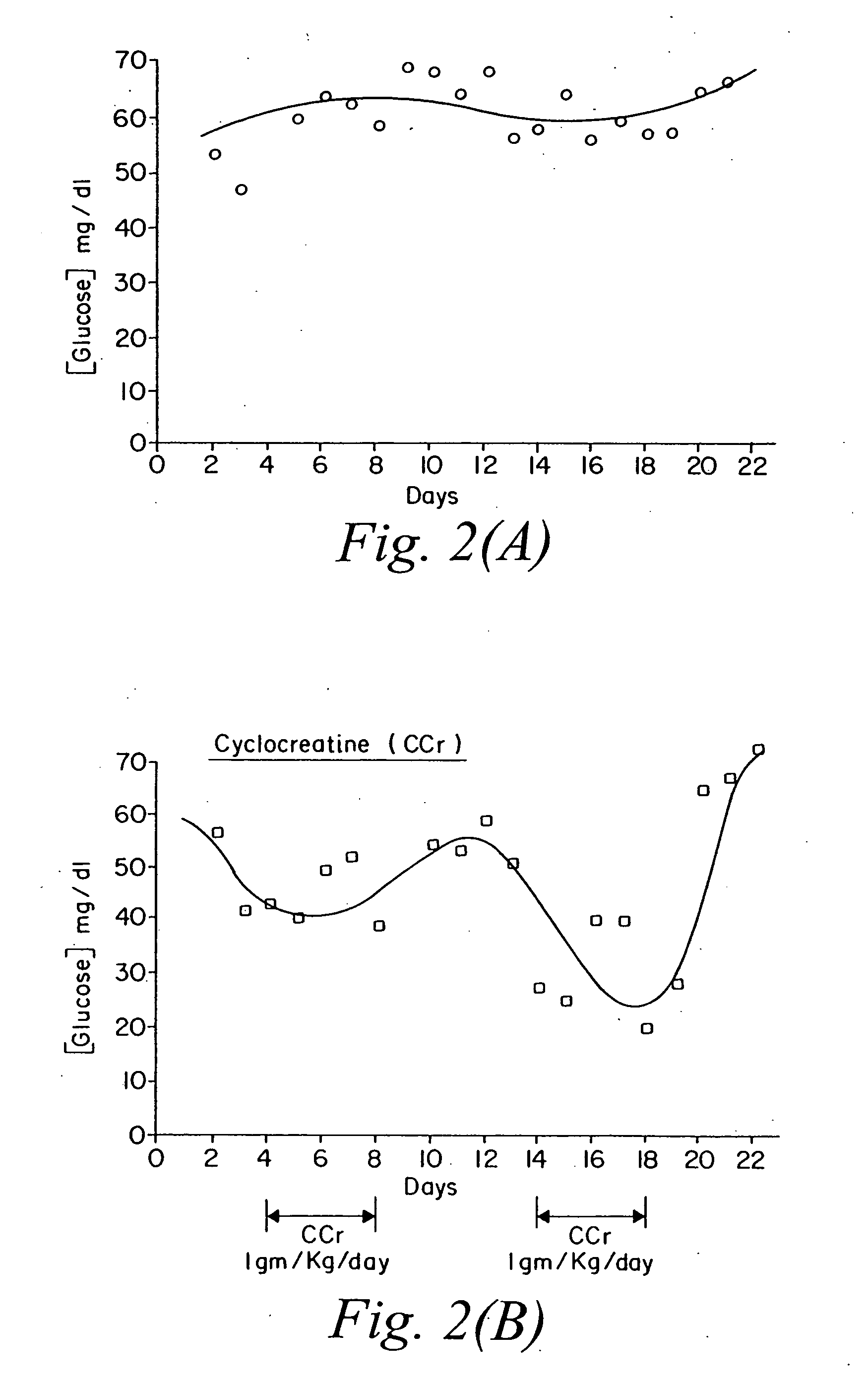
[0074] The same experiment described above was repeated. FIG. 2 shows the effect of the selected compounds on glucose levels. Panel (A): control (unmanipulated animals); Panel (B): cyclocreatine treated; Panel (C): beta-guanidino propionic acid treated; Panel (D): creatine phosphate treated animals. The same pattern seen in Example 1 is also seen here. Cyclocreatine induced a drop in the level of glucose after each administration. The drop in the second cycle was more dramatic than the first. Beta-guanidino propionic acid had minimal effect, and creatine phosphate seemed to mirror the effect of cyclocreatine.
example 3
Effect of Creatine Compounds on Glucose Levels in Rats Bearing Tumors
[0075] To examine more closely what occurred in the above two experiments, the average readings of glucose levels from experiments one and two were taken in the following time intervals post drug treatment: Days 2-3, Days 4-8, Days 8-12, Days 14-18, Day 15 and Days 19-22. Day 15 demonstrates the largest effect on glucose levels by this class of compounds. FIG. 3 outlines these results. Cyclocreatine, Panel (A), shows a drop in glucose level that could be as high as 50% on day 15. Beta-guanidino propionic acid, Panel (B), shows minimal effects <15%, and creatine phosphate, Panel (C), seems to drop glucose levels by 35% on day 15.
[0076] The experiments described above demonstrate that creatine analogues which modulate the creatine kinase system, and that are represented by cyclocreatine and creatine phosphate, can regulate glucose levels. The creatine kinase enzyme system creatine kinase emerges as a novel target f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com