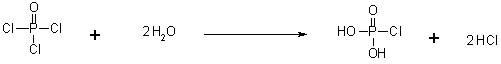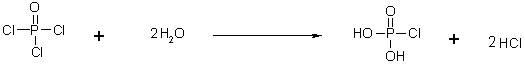Creatine phosphate sodium preparation method
A technology of sodium creatine phosphate and chlorophosphoric acid, which is applied in chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as high production costs, cumbersome post-processing, and harmfulness of barium. Achieve the effects of low production cost, simple post-processing, and pollution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] a) In the reaction flask equipped with sodium hydroxide absorption device, add phosphorus oxychloride POCl 3 (153.33g, 1.0mol), cool down in an ice-water bath to 0°C~5°C, slowly add deoxygenated water (36g, 2.0mol) dropwise, after the addition, control the temperature of the reaction solution at 5°C~10°C and stir for 2 hours. Airtight storage for later use;
[0048] b) In the reaction flask equipped with sodium hydroxide absorption device, add S-methylisothiourea (60.0g, 0.67mol), N,N-dimethylformamide DMF 600mL, stir well and add the above prepared Chlorophosphoric acid 1mol, control the temperature of the reaction solution at 35°C~40°C and stir for 5 hours, evaporate N,N-dimethylformamide under reduced pressure to obtain phosphorylated S-methylisothiourea (114g, 0.67mol). The intermediate of this step reaction does not need to be purified in full amount to continue to do it;
[0049] c) In the reaction flask equipped with a sodium hydroxide absorption device, add th...
Embodiment 2
[0052] a) In the reaction flask equipped with sodium hydroxide absorption device, add phosphorus oxychloride POCl 3 (153.33g, 1.0mol), cooled in an ice-water bath to 0°C~5°C, slowly added deoxygenated water (36g, 2.0mol) dropwise, after the addition, controlled the temperature of the reaction solution at 0°C~5°C and stirred for 3 hours. Airtight storage for later use;
[0053] b) Add S-methylisothiourea (45.0g, 0.50mol) and 450mL dimethyl sulfoxide into the reaction flask equipped with a sodium hydroxide absorption device, stir well and add 1mol of chlorophosphoric acid prepared above to control the reaction The solution was stirred at a temperature of 20°C to 30°C for 6 hours, and the dimethyl sulfoxide was distilled off under reduced pressure to obtain phosphorylated S-methylisothiourea (85.0 g, 0.5 mol). The intermediate of this step reaction does not need to be purified in full amount to continue to do it;
[0054] c) Add the phosphorylated S-methylisothiourea (85.0g, 0....
Embodiment 3
[0057] a) In the reaction flask equipped with sodium hydroxide absorption device, add phosphorus oxychloride POCl 3 (153.33g, 1.0 mol), cool down to 0°C~5°C in an ice-water bath, slowly add deoxygenated water (36 g, 2.0 mol) dropwise, after the addition, control the temperature of the reaction solution at 2°C~7°C and stir for 2.5 hours . Airtight storage for later use;
[0058] b) Add S-methylisothiourea (60.0g, 0.67mol) and 600mL of acetone to the reaction flask equipped with a sodium hydroxide absorption device, stir well and add 0.67mol of chlorophosphoric acid prepared above to control the temperature of the reaction solution Stir at 40°C~50°C for 2 hours, distill off the acetone under reduced pressure to obtain phosphorylated S-methylisothiourea (114g, 0.67mol). The intermediate of this step reaction does not need to be purified in full amount to continue to do it;
[0059] c) In the reaction flask equipped with a sodium hydroxide absorption device, add the phosphoryla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com