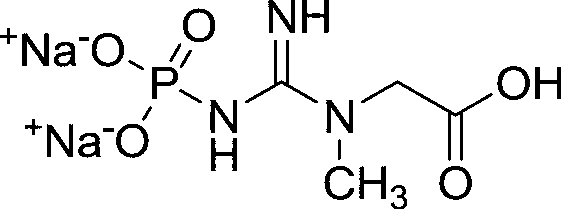
Crystal preparation method of creatine phosphate sodium
A creatine phosphate sodium crystallization technology, which is applied in the field of crystallization preparation of creatine phosphate sodium, can solve the problems of low single-pass yield, small particle size, and high production cost, and achieve uniform particle size distribution, high crystallinity, and complete crystal habit Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] At 20°C, dissolve the crude product of creatine phosphate in water to make an aqueous solution of creatine phosphate with a concentration of 0.8g / ml, add activated carbon whose mass is 2% of the mass of creatine phosphate, and continuously stir for 60 minutes to decolorize After filtration, the filtrate is moved into a crystallizer, the temperature of the system is controlled to 5°C, and acetone is added for elution and crystallization, and the time of dropping is controlled to be 2 hours, and the volume of acetone is twice that of water; then, filter and separate, and wash the filter cake with acetone, The obtained wet crystals were dried at 40° C. for 4 hours at an absolute pressure of 0.008 MPa. The HPLC purity of the final crystals was 99.61%, the primary particle size was 69.28 μm, and the single-pass molar yield in the crystallization process was 98.0%.
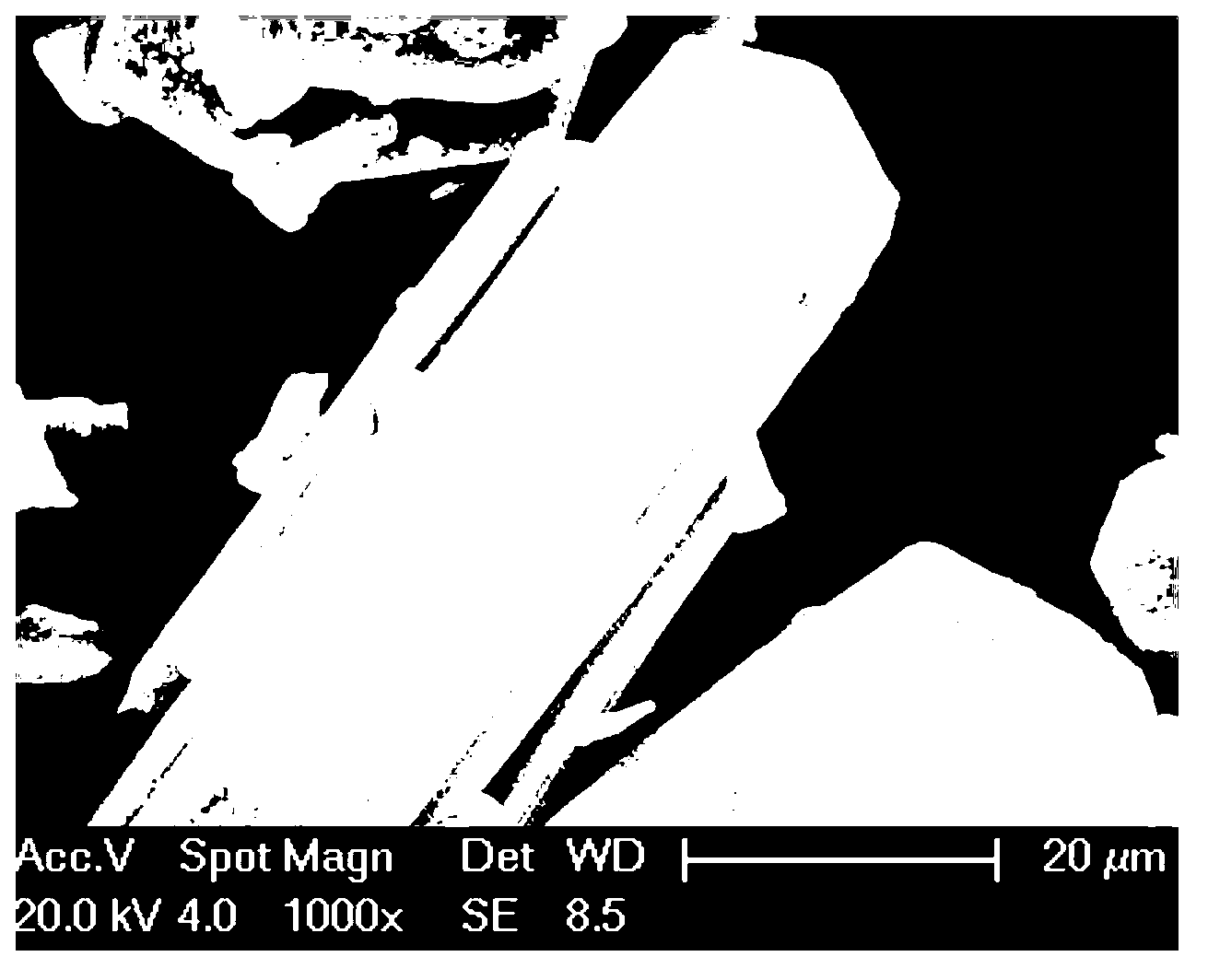
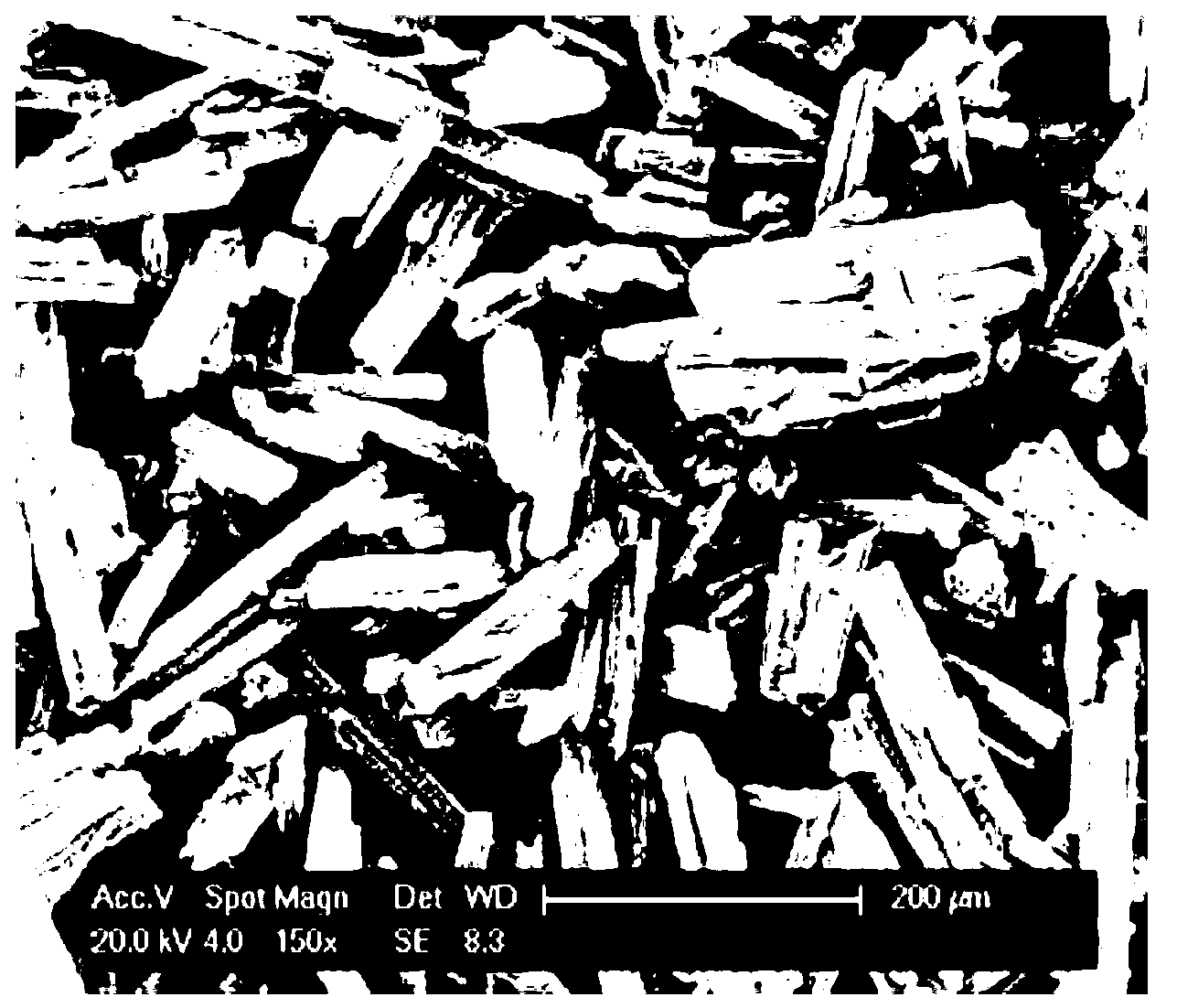
[0019] The scanning electron microscope picture of the obtained product is as follows: figure 1 , figure 2 ...
Embodiment 2
[0021] At 25°C, dissolve the crude product of creatine phosphate in water to make an aqueous solution of creatine phosphate sodium with a concentration of 1.1 g / ml, add activated carbon with a mass of 1% of the mass of creatine phosphate sodium, and continuously stir for 30 minutes for decolorization After filtration, the filtrate is moved into a crystallizer, the temperature of the system is controlled to 10°C, methanol is added for dissolution and crystallization, and the time of dropping is controlled to be 5 hours, and the volume of methanol is 5 times that of water; then, filter and separate, and wash the filter cake with ethanol, The obtained wet crystals were dried at 60° C. for 6 hours at an absolute pressure of 0.1 MPa. The HPLC purity of the final crystals was 99.50%, the primary particle size was 68.05 μm, and the single-pass molar yield in the crystallization process was 99.1%.
Embodiment 3
[0023] At 30°C, dissolve the crude creatine phosphate in water to make an aqueous solution of creatine phosphate sodium with a concentration of 0.5 g / ml, add activated carbon with a mass of 3% of that of creatine phosphate sodium, and continuously stir for 40 minutes for decolorization After filtration, the filtrate is moved into a crystallizer, the temperature of the system is controlled to 25°C, and ethanol is added for elution and crystallization, and the dropwise addition time is controlled to be 3 hours, and the volume of ethanol is 2.5 times that of water; then, filter and separate, and wash the filter cake with ethanol, The obtained wet crystals were dried at 40° C. for 2 hours at an absolute pressure of 0.02 MPa. The HPLC purity of the final crystals was 99.58%, the primary particle size was 68.35 μm, and the single-pass molar yield in the crystallization process was 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com