Preparation technique of sodium phosphocreatine powder and injection preparation
A preparation technology of creatine phosphate sodium, which is applied in the direction of medical preparations containing active ingredients, powder transportation, freeze-drying transportation, etc., can solve the problems of poor clarity and easy contamination, and achieve good clarity and easy operation. Simple and suitable for large-scale production
Inactive Publication Date: 2008-10-22
上海慈瑞医药科技股份有限公司
View PDF0 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
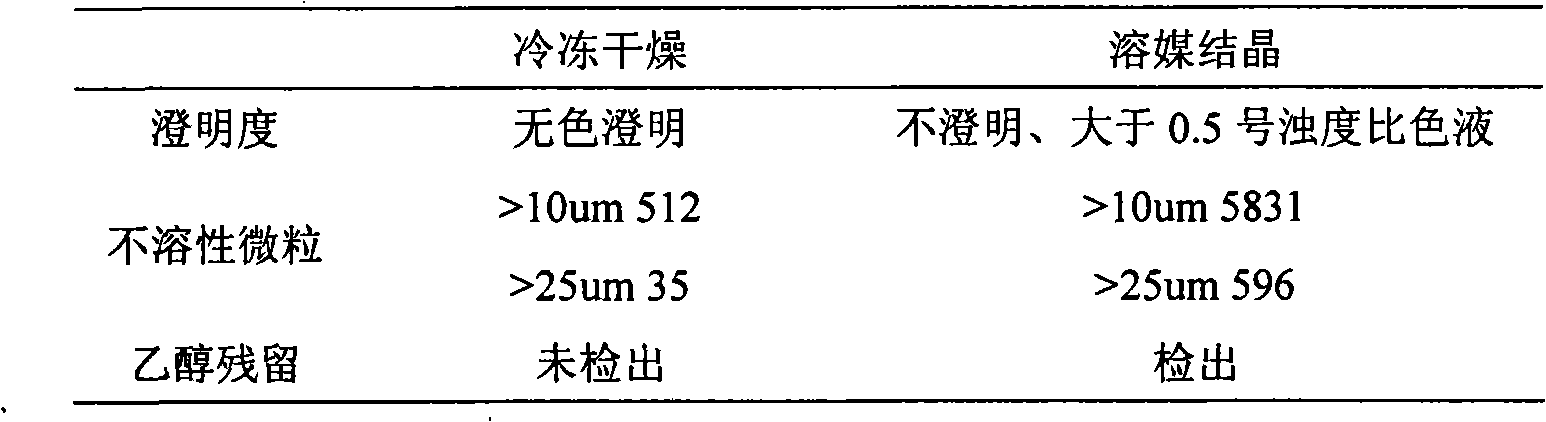
Sodium creatine phosphate is easily soluble in water. The current preparations are mainly powder injections for injection. The production process adopts solvent crystallization, which is usually the method of dissolving in water, adding absolute ethanol to crystallize, and directly aseptically subpackaging. This method of production Creatine phosphate sodium powder injection often has problems such as poor clarity and easy contamination
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a preparation process of a creatine phosphate sodium powder injection, which is characterized in that, the freeze-drying process is adopted for preparation, the process is that: 1 part of the formulated amount of creatine phosphate sodium is taken, 3 to 6 parts of water for injection is added, the creatine phosphate sodium is dissolved by stirring, 0.1 percent of the solution amount of activated carbon with needle use is added, the filtration for carbon removal and sterilization is carried out, thus forming clarified liquid, the water for injection is supplemented till the sufficient amount, a micro-porous membrane of 0.22Mum is used for filtration, and the filling is carried out after the content is 90 percent to 110 percent by measurement; the freeze-drying and the cover pressing are carried out; the well filled injection is arranged in a freeze-drying machine, the pre-freezing is firstly carried out till minus 50 DEG C to minus 35 DEG C, the temperature is kept for 1 to 5 hours, the vacuum pumping is carried out, the temperature raises to minus 10 DEG C to minus 3 DEG C within 15 to 30 hours, the temperature further raises to 15 to 50 DEG C within 2 to 10 hours, and the temperature is continuously kept for 3 to 10 hours, thus obtaining the creatine phosphate sodium powder injection. The preparation process has the advantages that the preparation process does not adopt ethanol, the clarity of the injection liquid is good, the operation is simple and the preparation process is applicable to mass production.
Description
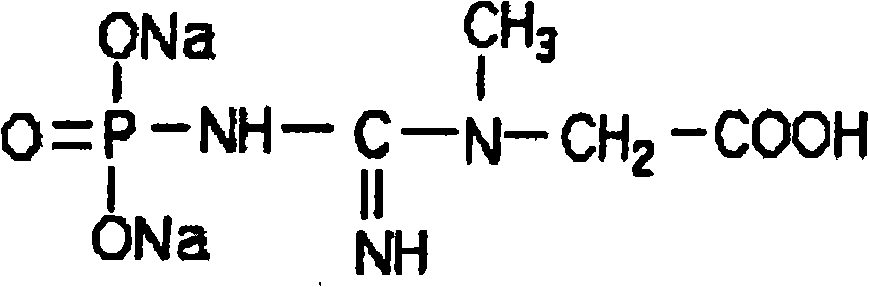
A kind of preparation technology of creatine phosphate sodium powder injection technical field The invention relates to a preparation process of creatine phosphate sodium powder injection, which can be used for myocardial protection and belongs to the technical field of medicines. Background technique Creatine Phosphate (CP) is an active substance in the human body and an important source of energy for the human body. It replenishes energy for ATP, the most important energy source in the process of cell metabolism. CP widely exists in various tissues in the body, and the content in muscle tissue is the highest, reaching 90% of the total amount in the body. Pharmacokinetic evidence shows that heart, skeletal muscle, brain and kidney can absorb exogenous CP. Its structural formula is: Studies have found that creatine phosphate has important pharmacological effects, especially its sodium salt compound creatine sodium phosphate has been widely used in clinical practice. A...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/19A61K31/664A61P9/00
Inventor 金幸汤磊朱益锋任兴发
Owner 上海慈瑞医药科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com