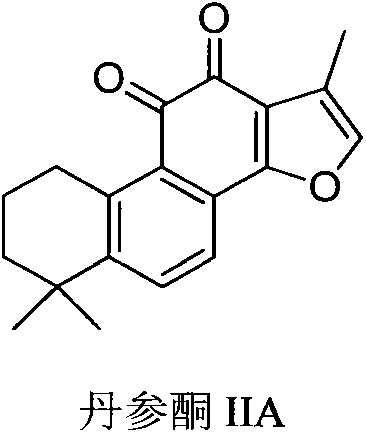
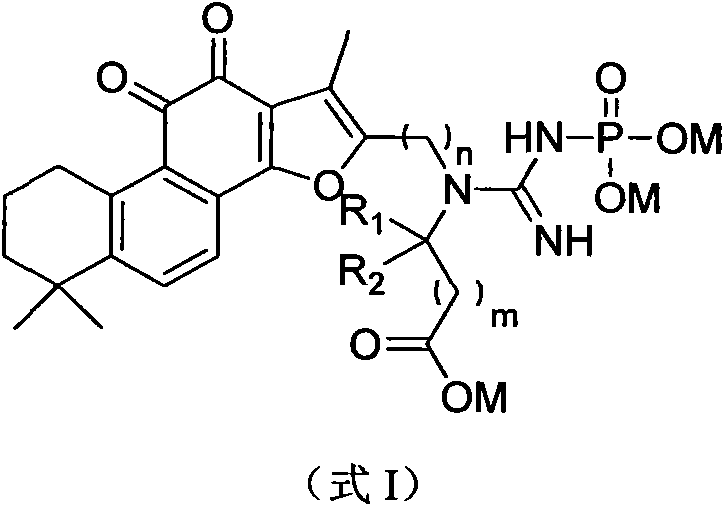
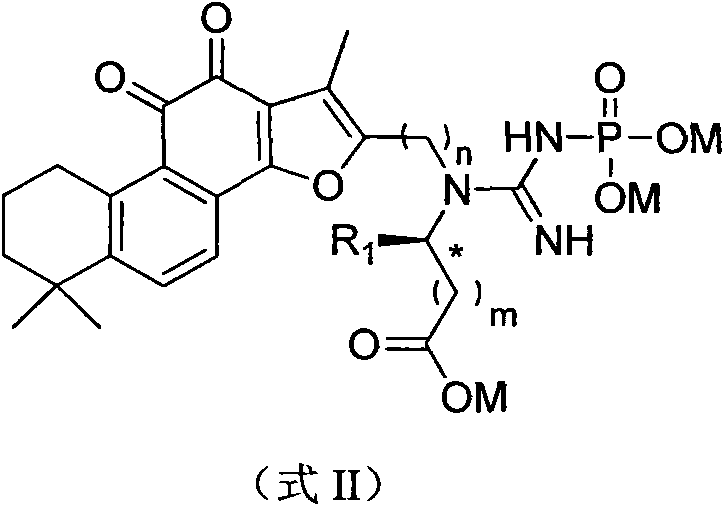
Synthesis and pharmaceutical application of sulfamide derivative
A technology of pharmacy and prodrug, applied in the field of medicine, can solve the problems of patient suffering, poor stability, easy desulfonation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] [Example 1] 2-(1-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2- b] furan-2-yl)methyl-3-phosphonoguanidino)acetic acid Preparation of trisodium
[0072] Step 1: Preparation of Tanshinone IIA-2-carbaldehyde
[0073] Tanshinone IIA (30 g, 0.01 mol) was dissolved in 300 mL of DMF, 30 mL of phosphorus oxychloride was added dropwise at room temperature, and stirred at room temperature for 2 hours. After the reaction, the reaction solution was poured into 1000 mL of ice-water mixture, stirred slowly, and a pale yellow solid was precipitated, filtered with suction, and the filter cake was washed with cold water three times, 100 mL each time. The filter cake was collected and vacuum-dried to obtain 32 g of tanshinone IIA-2-carbaldehyde (light yellow solid), and the yield was quantitative. 1 H NMR (400MHz, CDC13), 9.85(s, 1H), 7.78-7.69(d and d, 2H), 3.19(m, 2H), 1.80(m, 2H), 1.67(m, 2H), 2.65(s , 3H), 1.31 (s, 6H). LCMS (ESI) m / z, 323 (M+1) +
...
Embodiment 2
[0082] [Example 2]: (2S)-2-(1-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene [1,2-b]furan-2-yl)methyl-3-phosphonoguanidine) Preparation of trisodium propionate
[0083] According to the preparation method in Example 1, using L-alanine methyl ester instead of glycine methyl ester as raw material, (2S)-2-(1-(1,6,6-trimethyl-10,11-di Trisodium oxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b]furan-2-yl)methyl-3-phosphonoguanidino)propionate. 1 H NMR (400MHz, D 2 O) δ7.75-7.73(m, 2H), 4.32(m, 1H), 3.48(s, 2H), 3.05(m, 2H), 2.04(s, 3H), 1.70(m, 2H), 1.61( m, 2H), 1.35(d, 3H) 1.21(s, 6H), LCMS m / z=518.14(M+1) +
Embodiment 3
[0084] [Example 3]: (2S)-2-(1-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexaoxophenanthrene [1,2-b]furan-2-yl)methyl-3-phosphonoguanidine base)-3-methyl-trisodium butyrate
[0085] According to the preparation method in Example 1, using L-valine methyl ester instead of glycine methyl ester as raw material, (2S)-2-(1-(1,6,6-trimethyl-10,11-di Oxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b]furan-2-yl)methyl-3-phosphonoguanidino)-3-methyl-butanoic acid Trisodium. 1 H NMR (400MHz, D 2 O) δ7.73-7.69 (m, 2H), 4.23 (m, 1H), 3.44 (s, 2H), 3.02 (m, 2H), 2.01 (s, 3H), 1.71 (m, 2H), 1.60 ( m, 2H), 1.77(m, 1H), 1.33(d, 3H), 1.20(s, 6H), 1.01(d, 6H).LCMS m / z=546.14(M+1) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com