Application of erucic acid in preparing medicine for preventing or treating vaccinum influenzae vivum
A technology of influenza A and influenza A virus, which is applied in the field of medicine, can solve the problems that the effect of erucic acid has not been reported, and achieve the effect of meeting the needs of large-scale production, not easy to drug resistance, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The inhibitory effect of embodiment one erucic acid on common influenza virus causing cytopathic
[0021] 1. Materials: cells and viruses
[0022] Low-generation MDCK cells (less than 25 generations) were purchased from ATCC and kept in our laboratory; A / PR / 8 / 34(H1N1), A / GZ / GIRD07 / 09(H1N1), A / HK / 8 / 68( H3N2), A / HK / Y280 / 97 (H9N2), B / Lee / 1940 (FluB) are preserved in our laboratory.
[0023] 2. Reagents:
[0024] DMEM / DF12 (1:1) medium was purchased from Gibco Company; MTT was purchased from Sigma Company, 5mg / mL MTT solution=50mg of MTT+100mL DMEM / DF12 (1:1) medium; virus culture solution (containing final concentration 1.5ug / mL TPCK trypsin): 100mL DMEM / DF12 (1:1) culture solution + 150uL 1mg / mL TPCK trypsin, ready to use; erucic acid is isolated from the traditional Chinese medicine Radix Isatidis as white powder, prepared The method is carried out with reference to the literature [Modern Medicine and Clinic, 2011, 26(5): 381-383], and can also be obtained through mar...
Embodiment 2
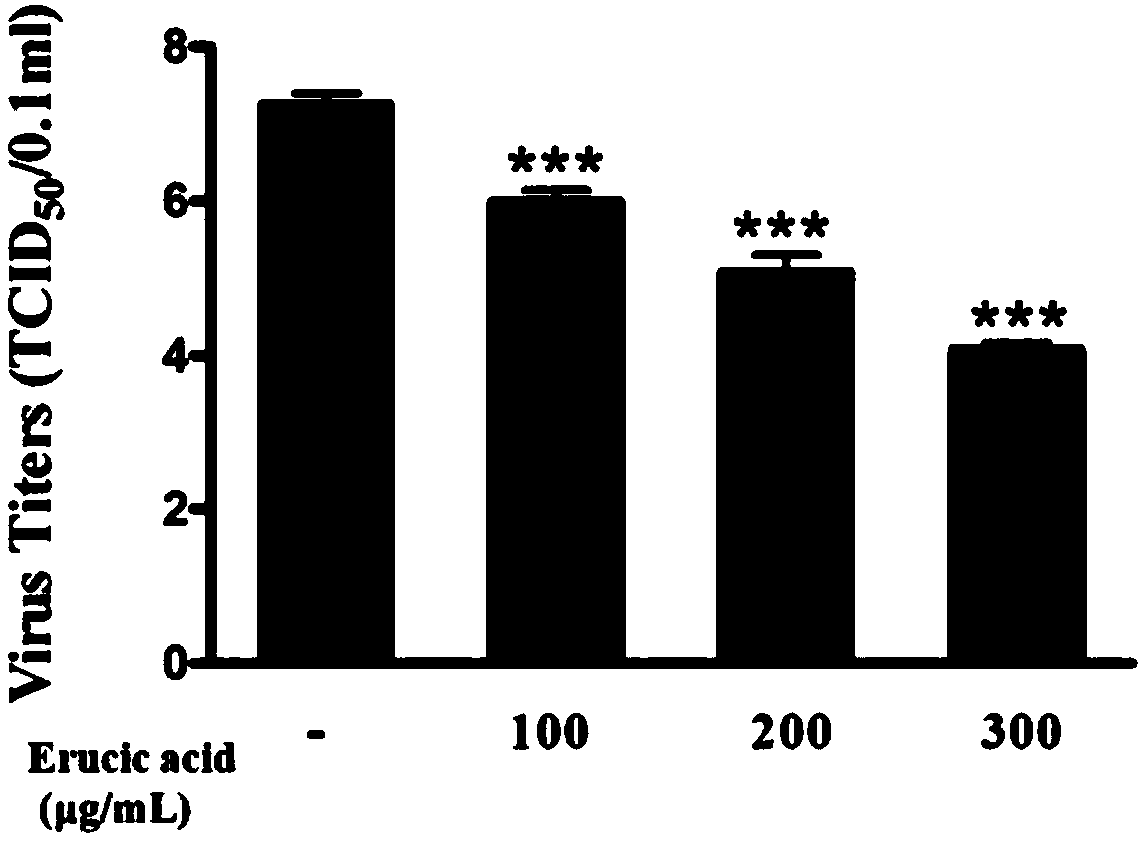
[0039] Embodiment dierucic acid is to the inhibitory action of influenza A / PR8 / 3 / 4 (H1N1) cell culture supernatant progeny virus
[0040] 1. Materials: cells and viruses
[0041] A549 cells were purchased from ATCC and kept in our laboratory
[0042] 2. Inhibitory effect of erucic acid on influenza A / PR8 / 3 / 4 (H1N1) progeny virus
[0043] 1) Discard the original medium of A549 cells in a single layer of 6-well plate, add PBS and wash twice;
[0044] 2) Add serum-free DMEM / DF12 (1:1) medium containing influenza virus A / PR8 / 3 / 4 (H1N1), and adsorb cells at 37°C for 2 hours;
[0045] 3) wash the cells with PBS to remove unadsorbed virus;
[0046] 4) The drug treatment mode was adopted. Different concentrations of erucic acid (100 μg / ml, 200 μg / ml, 300 μg / ml) were added to the drug intervention group, and 200 μg / ml erucic acid was added to the drug-only group. The A549 cells in the drug-only group were free from influenza. Virus adsorption, culture at 37°C for 24 hours;
[0047...
Embodiment 3
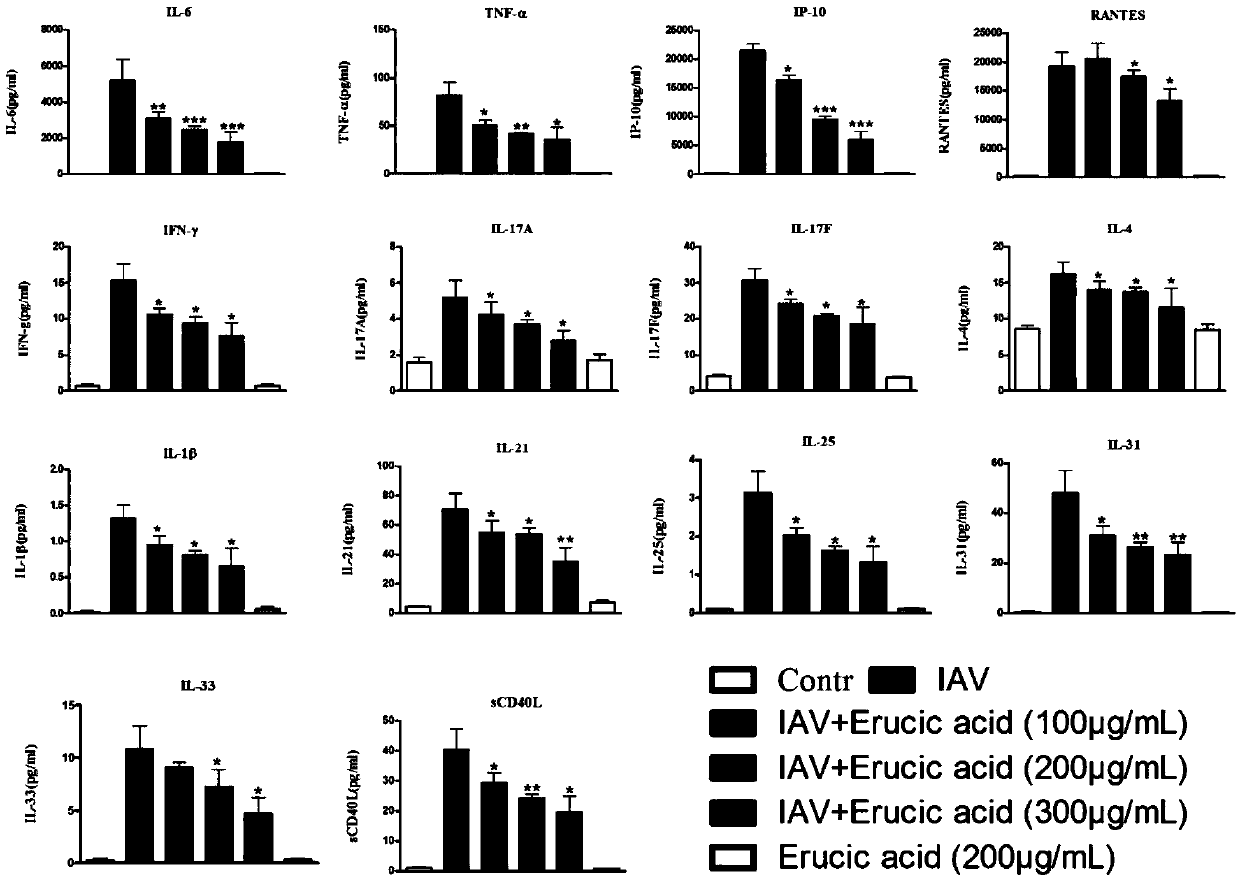
[0053] The effect of embodiment trierucic acid on influenza virus A / PR8 / 3 / 4 (H1N1) inducing host signaling pathway
[0054] 1. Chemical reagents and antibodies
[0055] Sodium chloride (NaCl), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), disodium hydrogen phosphate (Na2HPO4·12H2O), glycine Glycine, and Tween Tween-20 were all purchased from Guangzhou Chemical Reagent Factory; Tris base purchased from Ameresco; sodium dodecylsulfonate (SDS) was purchased from Sigma, USA; ammonium persulfate (APS) was purchased from Ameresco, USA; TEMED (N,N-methylenebisacrylamide) was purchased from Bio -rad company; RIPA lysate was purchased from American Thermo Company; PMSF was purchased from American Sigma Company; Cocktail (protease inhibitor) was purchased from American Sigma Company; BCA protein concentration assay kit was purchased from American Thermo Company; Easysee Western marker (20- 90kDa) was purchased from China TransgenBiotech Company; Blue plus II protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com