Chinese medicinal preparation for treating virus B hepatitis and preparation method thereof
A technology of viral hepatitis and traditional Chinese medicine preparations, which is applied in the direction of antiviral agents, medical formulas, and medical preparations containing active ingredients, etc., which can solve the problems of chemical ingredients that cause great harm to the human body, high cost of drug production, and large toxic and side effects. Enhance the immune function of the body, reduce the cost of treatment, and eliminate the effect of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation method of the tablet of the present invention comprises: taking 1500g of Cottonia japonicus, 1500g of Hedyotis diffusa, 1500g of Peilan, 1500g of Gardenia, 1500g of Bupleurum, 1500g of capillary, 1500g of Alisma, 1500g of Forsythia, 1500g of Radix Radix, 1500g of Poria cocos, Prunella vulgaris 1500g, Schisandra 1500g, Citrus aurantium 1500g, Atractylodes macrocephala 1500g, Cornus officinalis 1500g, Ligustrum lucidum 1500g, Radix Paeoniae Alba 1500g, Scutellaria baicalensis 1500g, Curcuma 1500g, Perilla 1500g, Toosendan 1500g, Curcuma 1500g, Gentiana 1500g, the The components are mixed according to the ratio, put into a pulverizer and crushed into fine powder, and the fine powder is sieved and put into an acid and alkali resistant impregnation pot. Squeeze and filter, take the filtrate after separation, heat and concentrate until it becomes a paste, mix it with starch, put it in an oven, control at 100°C, and press it into tablets.
[0054] The preparati...
experiment example 2
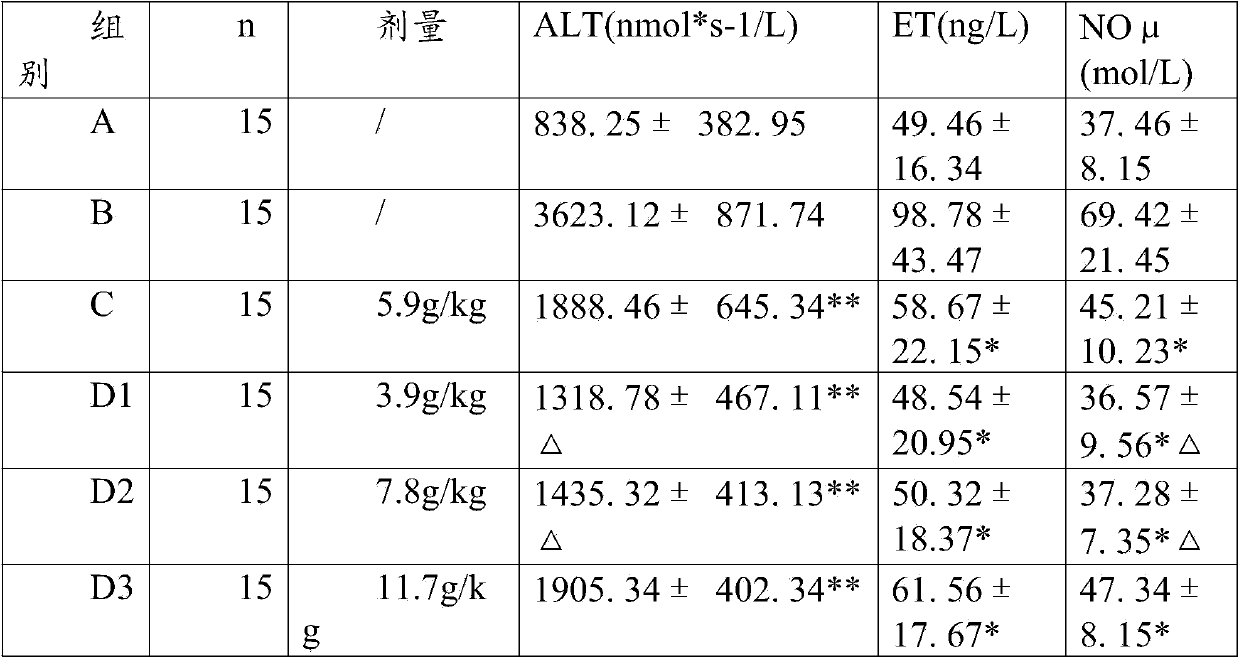
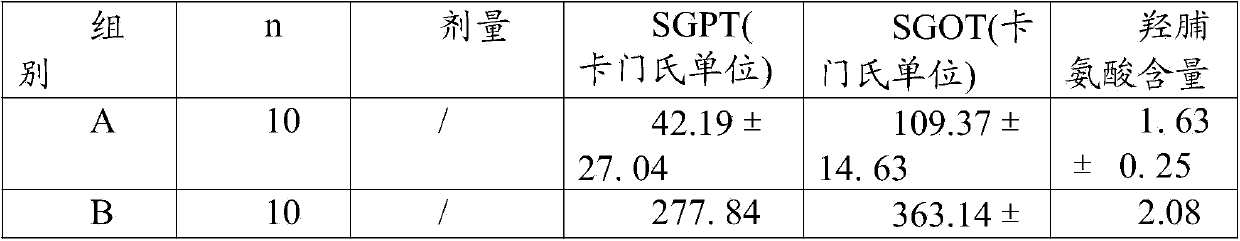
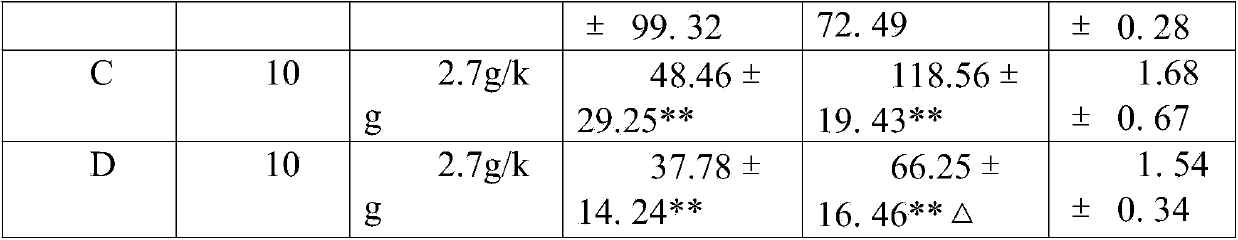
[0065] Experimental example 2: long-term toxicity test data
[0066] Capsule of the present invention is through gavage administration animal long-term toxicity test result of 6 months: SD rat is divided into three groups, every group of 30, male and female (divided into high, medium and low three dosage groups, every group 10 Only), wherein the first group is given normal saline through stomach gavage; the dose of the present invention is 0.25g, 2.5g and 12.5g / kg.d for the second group through stomach gavage; the third group is given lovastatin through stomach gavage The doses are 0.5mg, 5.0mg and 50.0mg / kg.d. During the administration period, the clinical manifestations, hematology, blood biochemistry, organ coefficient and histopathology of the animals in each group after administration were observed to evaluate the drug toxicity. Research results: The main toxic reaction was manifested in the lovastatin 50mg / kg.d and 5.0mg groups. After administration, the SD rats had sal...
Embodiment 1
[0135] Example 1: Jia, male, 38 years old, the patient began to suffer from acute viral hepatitis (jaundice type) 3 years ago, and the liver function was significantly damaged. Due to a large amount of glucose input, he developed diabetes and was hospitalized for nearly two years. The function often fluctuates, and the liver function is obviously abnormal in the past 1 month. The examination before the visit: alanine aminotransferase 372U, musk turbidity 18U, fasting blood sugar 190mg%, urine sugar (+++), dry mouth, bitter taste, yellow urine, two Pain in hypochondriac, sometimes with epistaxis, yellow tongue coating, red edge, stringy and thready pulse. Taking the present invention, the side is used: 12g of Hedyotis diffusa, 11g of Hedyotis diffusa, 12g of Peilan, 13g of Gardenia, 12g of Bupleurum, 13g of capillary, 12g of Alisma, 14g of Forsythia, 12g of Radix Isatidis, 11g of Poria cocos, 12g of Prunella vulgaris , Schisandra 13g, Citrus aurantium 12g, Atractylodes macrocep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com