Sclerotiorin derivatives and their preparation methods and their application as anti-h1n1 influenza virus agents
A technology of derivatives and type A, applied in the field of sclerotiorin derivatives and their preparation, can solve the problems that no natural compound or its derivatives have anti-type A H1N1 influenza virus agents, etc., achieve strong inhibitory activity and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] (1) The culture of the endophytic fungus Penicillium sp. (TA33-1) of Gorgonian coral
[0014] The culture medium used for strain culture contains glucose 1.0% (percentage by weight, the same below), yeast extract 0.2%, peptone 0.2%, agar 1.0%, sodium chloride 3.0%, and the rest is water. Strains were cultured at 30°C for 3 days.
[0015] (2) Fermentation of the endophytic fungus Penicillium sp. (TA33-1) from Gorgonian coral
[0016] The culture medium used in the fermentation culture contains 40.0% rice (percentage by weight, the same below), 3.0% sodium chloride, and the rest is water; the fungal strain is cultured at 28° C. for 30 days.
[0017] (3) Extraction and separation of (+)-Sclerotiorin
[0018] Get 60 bottles of mycelia obtained in step (2), extract 3 times with chloroform-methanol mixed solution (1:1) and concentrate under reduced pressure, then extract 3 times with ethyl acetate to obtain crude extract; Extraction extract is carried out normal phase sili...
Embodiment 2
[0020] (1) The culture of the endophytic fungus Penicillium sp. (TA33-1) of Gorgonian coral
[0021] The culture medium used for strain cultivation contains 0.1%-5.0% of glucose (percentage by weight, the same below), 0.01%-1% of yeast extract, 0.01%-1% of peptone, 0.1%-3.0% of agar, and 0.05% of sodium chloride- 5%, and the rest is water. When used, it is made into a test tube slant, and the fungal strains are cultivated at 0-30°C for 3-15 days.
[0022] (2) Fermentation of the endophytic fungus Penicillium sp. (TA33-1) from Gorgonian coral
[0023] The culture medium used in the fermentation culture contains 1.0%-80.0% (weight percentage, the same below) of rice, 0.05%-5% of sodium chloride and the rest is water, and the fungal strain is cultivated at 0-30°C for 10-60 days.
[0024] (3) Extraction and separation of (+)-Sclerotiorin compound
[0025] Take 10-300 bottles of the obtained mycelium obtained in step (2), extract the obtained mycelium with chloroform-methanol mix...
Embodiment 3
[0028] Weigh the dried (+)-Sclerotiorin (0.1mol) and dissolve it in dichloromethane. Under normal temperature, add the organic primary amine (0.12mol) dropwise to the reaction solution under full stirring, and react for 1 hour Finally, add distilled water (200mL) to the reactant to terminate the reaction, extract with ethyl acetate (500mL), concentrate the extract, perform column chromatography, elute with ethyl acetate, concentrate the eluate, and recrystallize After obtaining the compound of formula I.
[0029] Other organic chemical reaction conditions not specifically specified in Example 3, and other experimental operating conditions such as normal phase silica gel column chromatography separation are all conventional experimental operating conditions in this field, and those skilled in the art can make reasonable choices according to actual needs .
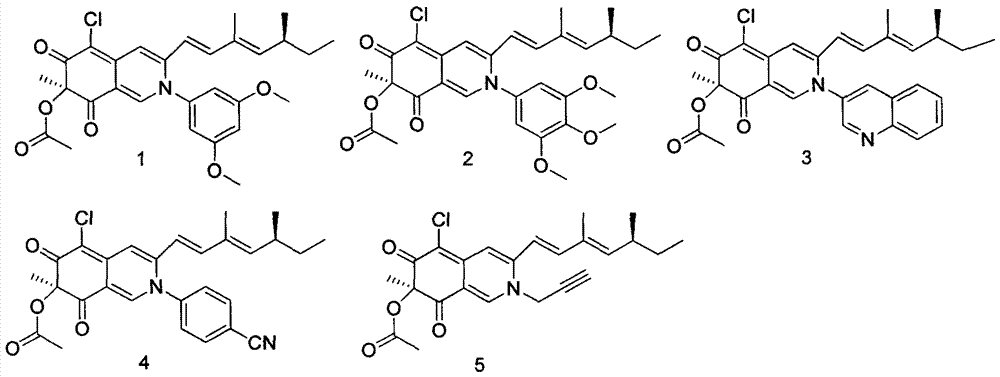
[0030] Specific compound structure examples of compounds of formula I:
[0031]
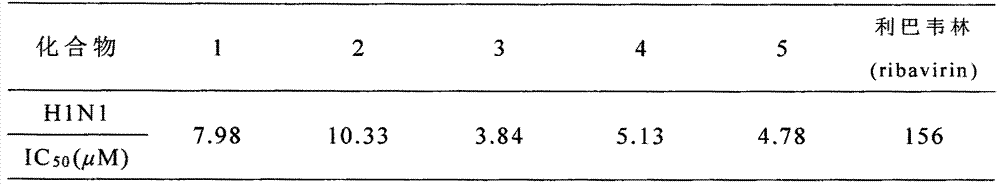
[0032] The structural confirmation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com