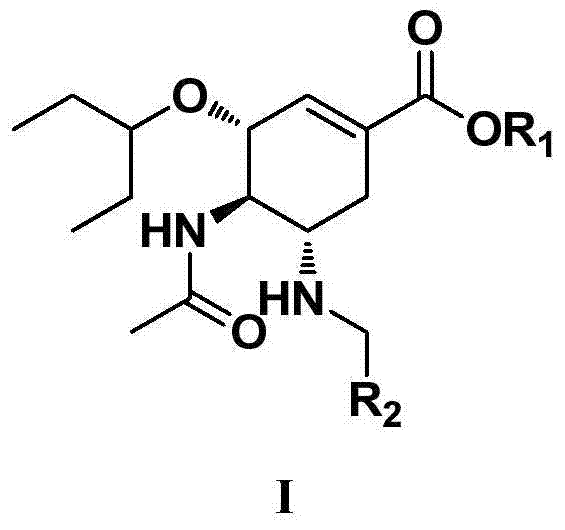
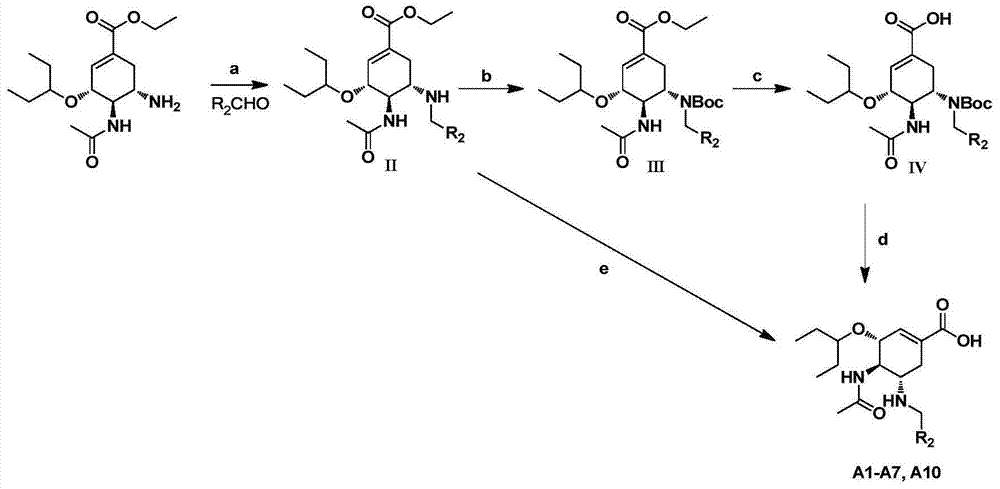
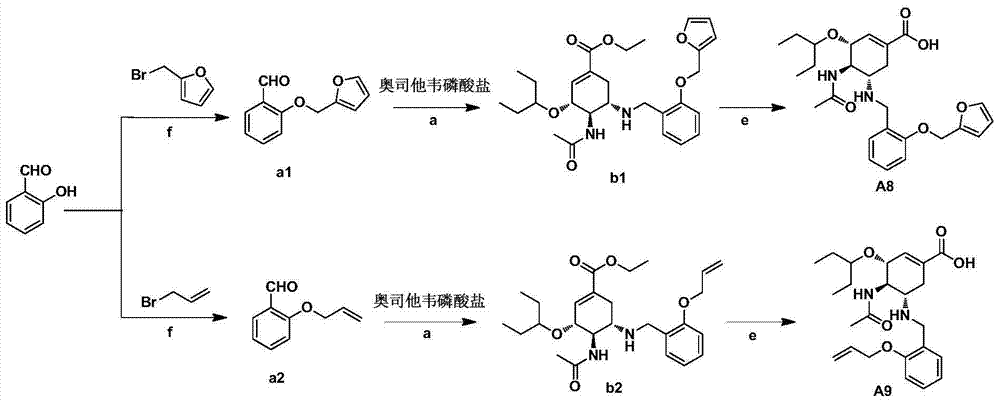
Oseltamivir derivative as well as preparation method and application thereof
A technology of oseltamivir and derivatives, which is applied in the field of disease drugs, oseltamivir derivatives and their preparation, can solve the problems of decreased effectiveness of NA inhibitors, limited clinical application and the like, and achieves highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (3R,4R,5S)-4-Acetamido-5-(([1,1'-biphenyl]-4-ylmethyl)amino)-3-(1-ethylpropoxy)-1 -Ethyl cyclohexene-1-carboxylate (II-10)
[0075] Add oseltamivir phosphate (0.82g, 2.0mmol) and 25.0mL absolute ethanol into a 50mL round bottom flask, add p-phenylbenzaldehyde (0.4g, 2.2mmol) under stirring conditions, stir at room temperature for 5 minutes, add Sodium cyanoborohydride (0.25g, 4.0mmol), react at room temperature. After four hours, filter through celite. Silica gel column chromatography gave 0.64 g of a white solid, with a yield of 67%. 1 H NMR (CDCl 3 ,300MHz)δ7.53-7.60(m,4H),7.30-7.46(m,5H),6.80(s,1H),5.50(d,1H,J=7.2Hz),4.18-4.26(m,3H) ,3.93-3.98(m,1H),3.72-3.82(m,2H),3.33-3.41(m,1H),3.15-3.24(m,1H),2.80(dd,1H,J=17.7,5.1Hz) ,2.22-2.35(m,1H),2.01(s,3H),1.46-1.56(m,4H),1.30(t,3H,J=7.2Hz),0.90(t,6H,J=7.5Hz). 13 C NMR (CDCl 3 ,75MHz)δ170.65,166.52,140.92,139.95,139.29,137.19,129.38,128.75,128.57,127.35,127.17,127.03,81.77,74.53,60.88,55.81,53.60,50.25,30.41,26.15,25....
Embodiment 2
[0080](3R,4R,5S)-4-Acetamido-5-((2-thienylmethyl)amino)-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid tris Fluoroacetate (A1)
[0081] The experimental operation is the same as in Example 1, the product II-1 obtained by the reaction of oseltamivir phosphate (0.82g, 2.0mmol), thiophene 2-carbaldehyde (0.25g, 2.2mmol) and sodium cyanoborohydride (0.25g, 4.0mmol) , a white solid was obtained after Boc protection, hydrolysis and de-Boc, the total yield was 27%, mp=86~88℃. 1 H NMR (DMSO-d 6 ,300MHz)δ7.80(d,1H,J=9.0Hz),7.37(dd,1H,J=4.5,1.8Hz),6.93-6.97(m,2H),6.59(s,1H),3.94-4.01 (m,2H),3.85-3.91(m,1H),3.66-3.76(m,1H),3.31-3.38(m,1H),2.71-2.80(m,1H),2.64(dd,1H,J= 17.4,4.8Hz),2.01-2.11(m,1H),1.91(s,3H),1.85(s,3H),1.33-1.49(m,4H),0.83(t,3H,J=7.5Hz), 0.79(t,3H,J=7.5Hz). 13 C NMR (DMSO-d 6 ,75MHz)δ177.22,174.83,172.84,150.35,142.52,134.61,131.77,129.74,129.56,86.06,80.58,59.59,59.17,49.78,35.63,30.85,30.39,28.23,26.27,14.65,14.17.HRMS calculated for calcd for C 19 h 29 N 2...
Embodiment 3
[0083] (3R,4R,5S)-4-Acetamido-5-(benzylamino)-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid trifluoroacetate (A2 )
[0084] The experimental operation was the same as in Example 1, the product II-2 obtained by the reaction of oseltamivir phosphate (0.82g, 2.0mmol), benzaldehyde (0.23g, 2.2mmol) and sodium cyanoborohydride (0.25g, 4.0mmol) was tested by Boc protection, hydrolysis and de-Boc yielded a white solid with a total yield of 52%, mp=80-82°C. 1 H NMR (CD 3 OD,300MHz)δ7.45-7.51(m,5H),6.90(s,1H),4.40-4.45(m,2H),4.18-4.30(m,3H),3.57-3.67(m,1H),3.42 -3.50(m,1H),3.05(dd,1H,J=17.4Hz,5.7Hz),2.62-2.73(m,1H),2.06(s,3H),1.49-1.60(m,4H),0.93( t,3H,J=7.2Hz),0.91(t,3H,J=7.2Hz). 13 C NMR (CD 3 OD,75MHz)δ173.02,166.58,136.78,130.25,129.01,128.85,128.48,126.69,81.86,74.00,54.32,50.94,25.25,25.21,24.71,21.41,7.88,7.63. 21 h 31 N 2 o 4 [M+H] + :375.2284,found:m / z375.2280.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com