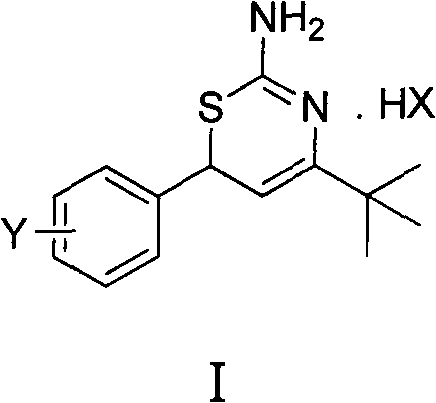
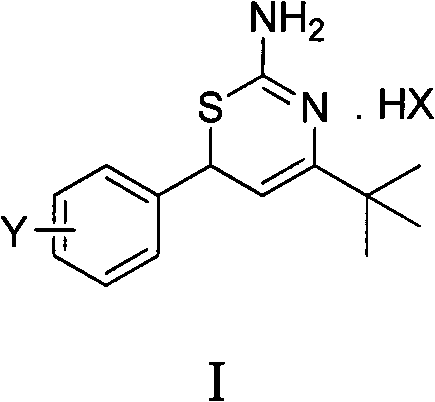
Preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt
A tert-butyl, -6H-1 technology, applied in the direction of effective components of heterocyclic compounds, antiviral agents, organic chemistry, etc., can solve the problems of effectiveness impact, helplessness of influenza virus strains, lack of understanding, etc., and achieve low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 4-tert-butyl-6-(4-chlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I a ) preparation
[0030]
[0031] 0.010mol 1-(4-chlorophenyl)-4,4-dimethylpenten-3-one and 0.012mol thiourea, 20mL ethanol, 0.011mol hydrochloric acid were stirred and refluxed, the reaction was detected by TLC, and the reaction was completed for 5 hours. Washed with water, dried to obtain 4-tert-butyl-6-(4-chlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I a ). Yield 87.0%, mp 228-230°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.23(s, 9H, 3×CH 3 ), 5.05 (d, J=6.0Hz, 1H, thiazine ring 5-H), 5.17 (d, J=6.0Hz, 1H, thiazine ring 6-H), 7.26 (d, J=6.0Hz, 2H , benzene ring 2, 6-H), 7.35 (d, J=6.8Hz, 2H, benzene ring 3, 5-H), 9.74, 9.99 (2×s, 2H, NH 2 ), 11.3(s, 1H, N + h).
Embodiment 2
[0032] Example 2 4-tert-butyl-6-(2-chlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I b ) preparation
[0033]
[0034] 0.010mol 1-(2-chlorophenyl)-4,4-dimethylpenten-3-one and 0.012mol thiourea, 20mL ethanol, 0.011mol hydrochloric acid reacted at 30°C, TLC detected the reaction, reacted for 3h, and reacted Complete, wash with water, dry to get 4-tert-butyl-6-(2-chlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I b ), yield 65.0%, mp 149~150°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.33(s, 9H, 3×CH 3 ), 5.19 (d, J=5.6Hz, 1H, thiazine ring 5-H), 5.36 (d, J=6.0Hz, 1H, thiazine ring 6-H), 7.27~7.43 (m, 4H, benzene ring H), 9.03, 10.23 (2×s, 2H, NH 2 ), 11.59 (s, 1H, N + h).
Embodiment 3
[0035] Example 3 4-tert-butyl-6-(2,4-dichlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I c ) preparation
[0036]
[0037] 0.010mol 1-(2,4-dichlorophenyl)-4,4-dimethylpenten-3-one and 0.012mol thiourea, 20mL methanol, 0.011mol hydrochloric acid reflux, TLC detection reaction, reaction 6h, reaction Finished, washed with water, dried to obtain 4-tert-butyl-6-(2,4-dichlorophenyl)-2-amino-6H-1,3-thiazine hydrochloride (I c ), yield 52.1%, mp 105°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.32(s, 9H, 3×CH 3 ), 5.17 (d, J=5.2Hz, 1H, thiazine ring 5-H), 5.30 (d, J=4.4Hz, 1H, thiazine ring 6-H), 7.20~7.44 (m, 3H, benzene ring H), 9.28, 10.20 (2×s, 2H, NH 2 ), 11.54(s, 1H, N + h).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com