Schizandrin, schisanhenol and schisandrin-b derivates and application thereof
A technology of schisandrin A and schisandrin B, which is applied in the field of schisandrin and schisandrin derivatives, schisandrin A, can solve the problems of drug withdrawal rebound and achieve low price, significant protective effect, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
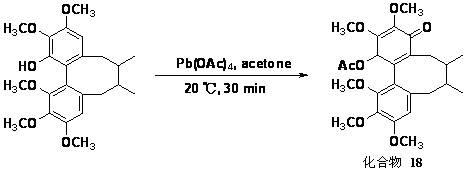
[0017] Embodiment 1: Schisandrin and Schisandrin C 4 、C 11 double chlorination
[0018] Weigh 0.25 mmol of schisandrin (or schisandrin) and 0.52 mmol of N-chlorosuccinimide (NCS) in a dry 25 mL round bottom flask, and then add 5 mL of methanol into the round bottom flask (analytical pure), stirred at room temperature for 1 h, added water to terminate the reaction, extracted three times with chloroform, and the chloroform layer was washed with Na 2 SO 4 Dry, filter, concentrate the organic layer, and prepare compound 1 (yield 44.4%) and compound 2 ( The yield is 98.4%).
[0019]
[0020] EI-MS: m / z 468 [M] + ; 1 H-NMR (CDCl 3 , 400 MHz) δ (ppm): 6.06 (2H, m), 3.94 (6H, s), 3.76 (3H, s), 3.56 (3H, s), 2.48 (1H, m), 2.27 (2H, m), 2.01 (1H, m), 1.77 (1H, m), 1.62 (1H, m), 1.04 (3H, d, J = 7.2 Hz), 0.84 (3H,d, J = 7.2 Hz); 13 C-NMR (CDCl 3 , 100 MHz) δ (ppm): 150.4(s), 149.4(s), 145.6(s), 144.7(s), 139.5(s), 135.0(s), 135.0(s), 133.0(s), 127.1(s), 124.6 (s), ...
Embodiment 2
[0023] Embodiment 2: Schisandrin and Schizandrin C 4 、C 11 double bromination reaction
[0024] Weigh 0.12 mmol of schisandrin (or schisandrin B) into a dry 25 mL round bottom flask, then add an appropriate amount of water-saturated CCl 4 solution, after the sample is dissolved, add 4 drops of bromine (Br 2 ), the mixed system was stirred at room temperature in the dark for 1 h, and then 10 % Na was added dropwise to the reaction system 2 S 2 o 3 , fully stirred until the color of bromine-free water, the reaction solution was extracted three times with chloroform, and the chloroform layer was extracted with Na 2 SO 4 Dry, filter, concentrate the organic layer, and prepare compound 3 (yield: 98%) and compound 4 by preparative thin layer chromatography (petroleum ether-ethyl acetate = (5-3): 1) (97.6% yield).
[0025]
[0026] EI-MS: m / z 560 [M] + ; 1 H-NMR (CDCl 3 , 400 MHz) δ(ppm): 6.05 (2H, m), 3.90 (6H, s), 3.73 (3H, s), 3.54 (3H, s), 2.46 (2H, m), 2.35 (2H...
Embodiment 3
[0029] Embodiment 3: Schisandrin A C 4 、C 11 double bromination reaction
[0030] Weigh 0.25 mmol of Schisandrin A and 0.62 mmol of N-chlorosuccinimide (NBS) respectively in a dry 25 mL round bottom flask, add 5 mL of methanol (analytical grade) into the round bottom flask, and store at room temperature The reaction was stirred for 1 h, extracted three times with water-chloroform, and the chloroform layer was washed with Na 2 SO 4 Dry, filter, concentrate the organic layer, and prepare compound 5 (95.9% yield) by preparative thin-layer chromatography (petroleum ether-ethyl acetate = 6:1 as the developing solvent).
[0031]
[0032] EI-MS: m / z 590 [M] + ; 1 H-NMR (CDCl 3 , 400 MHz) δ (ppm): 3.88 (12H, s), 3.52 (3H, s), 3.39 (3H, s), 3.08 (1H, m), 2.95 (1H, d, J = 14.0 Hz), 2.64 (2H, m), 1.88 (1H, m), 1.26 (3H, s), 0.82 (3H, d, J = 7.2 Hz); 13 C-NMR (CDCl 3 , 100 MHz) δ (ppm): 177.9(s), 150.8(s), 150.5(s), 150.3(s), 144.8(s), 144.4(s), 134.7(s), 133.3(s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com