Method for simultaneously detecting contents of four effective ingredients in antitussive tablet
A technology of active ingredients and content, which is applied in the field of simultaneous detection of four active ingredients in Zhikebao Tablets, can solve the problems of cumbersome experimental steps, poor control of experimental conditions, unsatisfactory repeatability and stability of the method, and achieve reproducible results. Good performance, simple and fast detection method, and the effect of improving the level of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
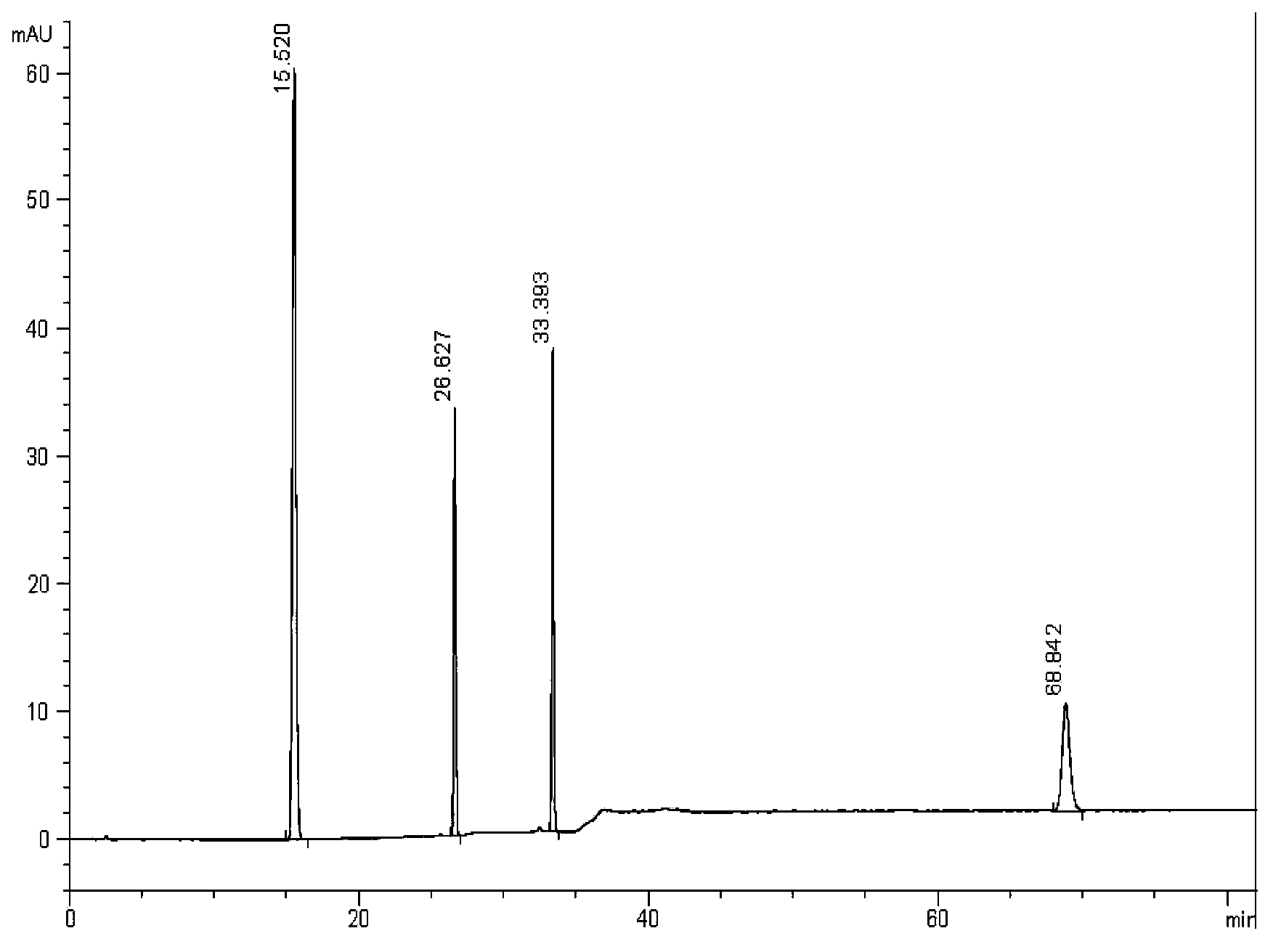
[0049] Simultaneously detect the content of schisandrin A, proseridin, prosertin and shionone in Zhikebao Tablets, the details are as follows (3 elution gradients):
[0050] 1. Experimental samples and reference substances
[0051] Zhikebao Tablets (manufactured by Guangdong Taicheng Pharmaceutical Co., Ltd., batch number: 20121101); dibassin reference substance (batch number: 111711-200602, provided by the China Institute for the Control of Pharmaceutical and Biological Products); , provided by China Institute for the Control of Pharmaceutical and Biological Products); Shivanone reference substance (batch number: 111581-200604, provided by China Institute for the Control of Pharmaceutical and Biological Products); .
[0052] 2. Instrument
[0053] High performance liquid chromatography Agilent1260Infinity, G1315D. Chromatographic column: Shiseido C18 (4.6×250mm, 5μm, column number: AKAD08072), one-hundred-thousandth grade electronic balance (model: 9ZSM-202A), ultrasonic i...
Embodiment 2
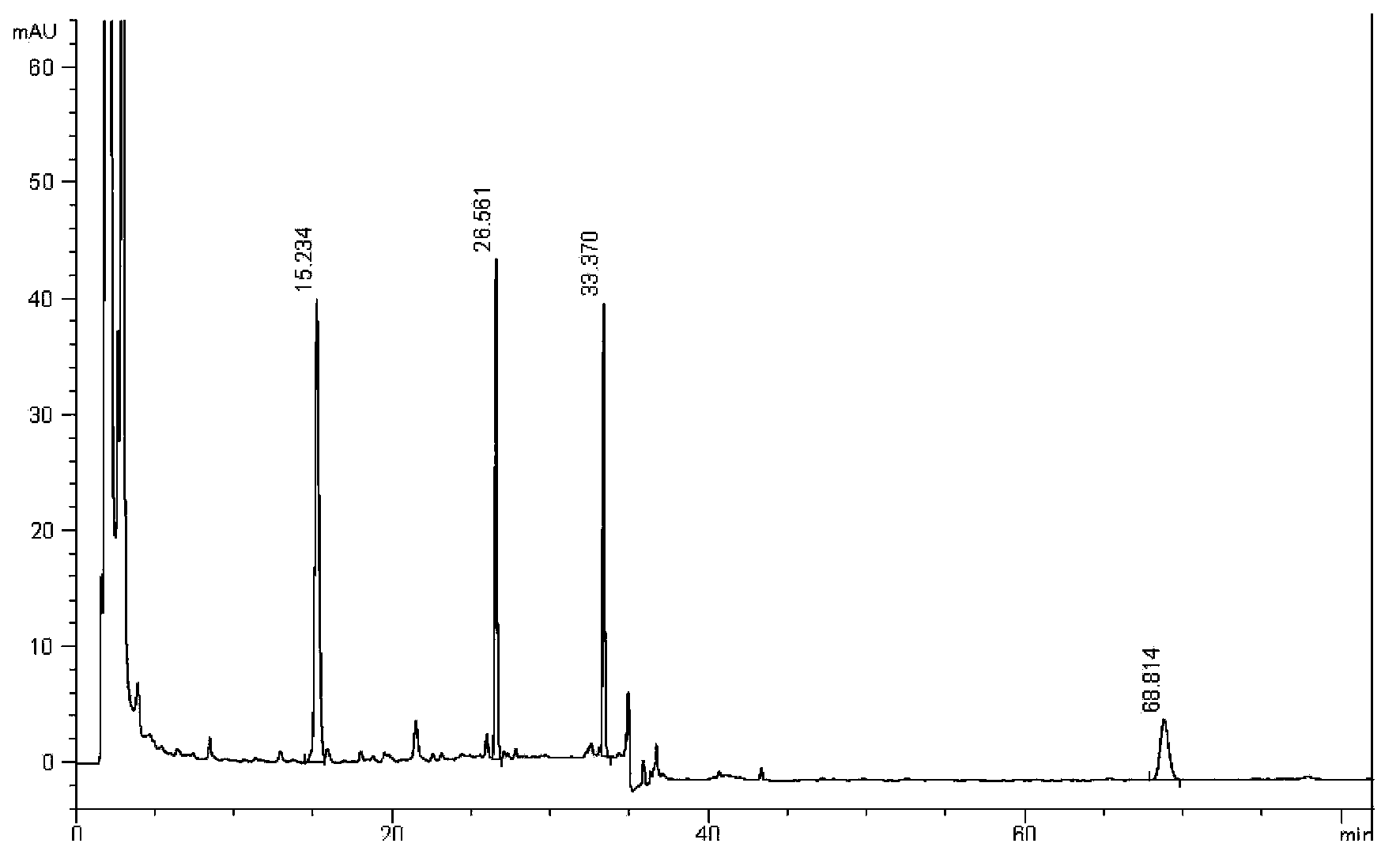
[0064] Simultaneously detect the content of schisandrin A, proseridin, prosertin and shionone in Zhikebao Tablets, the details are as follows (3 elution gradients):
[0065] 1. Experimental samples and reference substances
[0066] Zhikebao Tablets (produced by Guangdong Taicheng Pharmaceutical Co., Ltd., batch number: 20121102); others are the same as in Example 1
[0067] 2. Instrument
[0068] Same as Example 1
[0069] 3. Detection method
[0070] 3.1 Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A and water as mobile phase B, and carry out gradient elution according to the regulations in Table 2 (wherein, Gradient 1 is isocratic elution, gradient 2 is the gradient elution in which the volume percentage of mobile phase A and B changes continuously from 40%:60% isokinetic to 90%:10% during the elution process, and gradient 3 is isocratic elution off), using variable wavelength de...
Embodiment 3
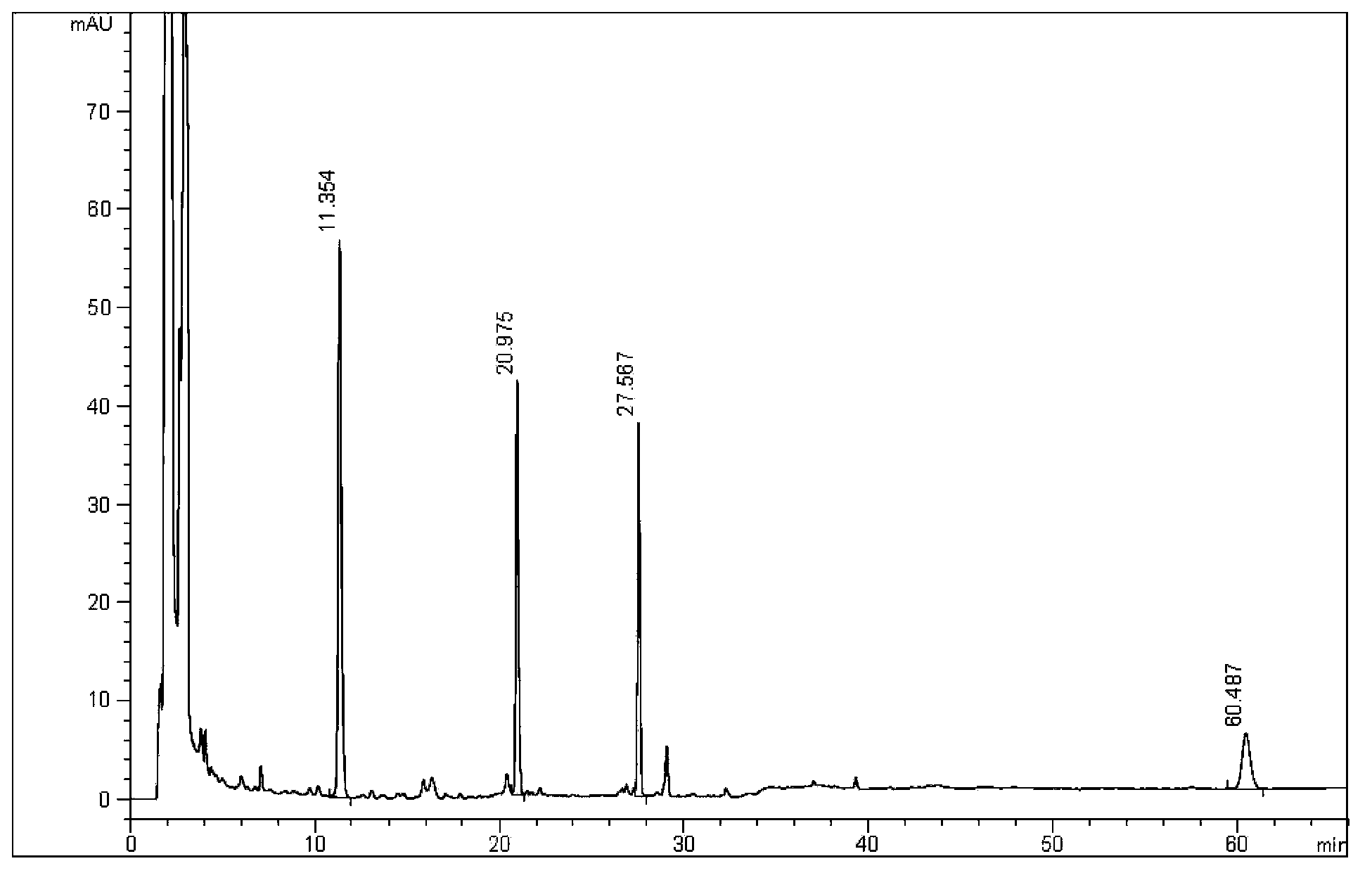
[0079] Simultaneously detect the content of schisandrin A, proseridin, prosertin and shionone in Zhikebao Tablets, the details are as follows (3 elution gradients):
[0080] 1. Experimental samples and reference substances
[0081] Zhikebao Tablets (produced by Guangdong Taicheng Pharmaceutical Co., Ltd., batch number: 20121103); others are the same as in Example 1
[0082] 2. Instrument
[0083] Same as Example 1
[0084] 3. Detection method
[0085] 3.1 Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A and water as mobile phase B, and carry out gradient elution according to the regulations in Table 3 (wherein, Gradient 1 is isocratic elution, gradient 2 is the gradient elution in which the volume percentage of mobile phase A and B changes continuously from 65%: 35% isokinetic to 100%: 0% during the elution process, and gradient 3 is isocratic elution off), using variable wavelength ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com