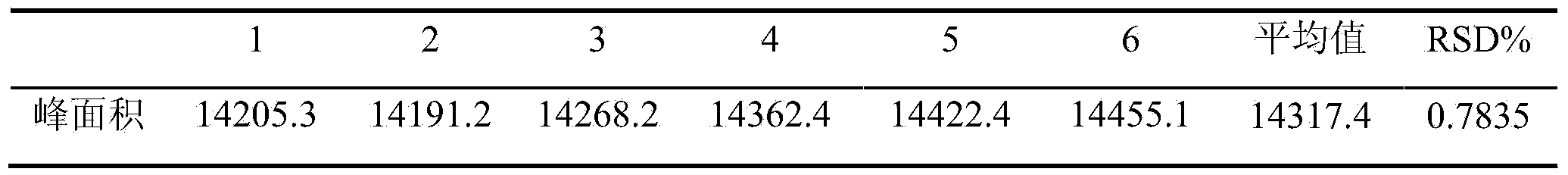
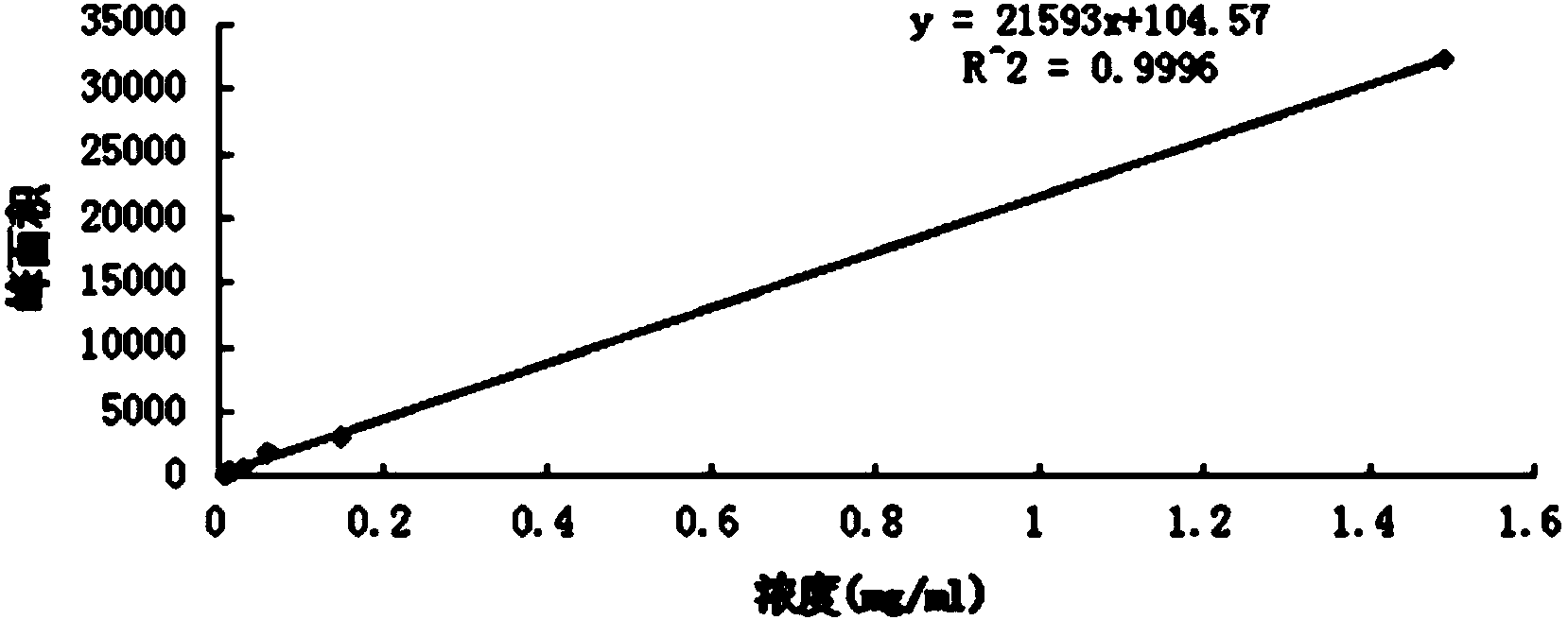Method for determining content of schisandrin in Yixinshu traditional Chinese medicine preparation
A technology for schisandrin A and traditional Chinese medicine preparations, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of unsuitable determination of schisandrin A in Yixinshu traditional Chinese medicine preparations, insufficient rigor of the determination method, and poor precision of instruments.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Determination of Schisandrin A in Yixinshu Capsules (commercially available):
[0060] (1) Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; methanol: water = 65:35 is used as mobile phase; flow rate is 1.0mL min -1 ;The detection wavelength is 250nm; the column temperature is 30°C; the number of theoretical plates should not be less than 2000 based on the peak of schisandrin A;
[0061] (2) Preparation of reference substance solution: Accurately weigh 3.02mg of schisandrin A reference substance, place it in a 10mL volumetric flask, add methanol to dissolve and set the volume to the mark, and prepare a solution with a concentration of 0.302mg / mL, pass through 0.22μm The microporous membrane, take the continued filtrate, to obtain final product;
[0062] (3) Preparation of the test solution: take 30 capsules of Yixinshu capsules and mix them evenly; take 1g, accurately weigh it, put it in a 25mL volumetric flask...
Embodiment 2
[0065] Determination of Schisandrin A in Yixinshu Granules (commercially available):
[0066] (1) Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; methanol: water = 75:25 is used as mobile phase; flow rate is 1.5mL min -1 ;The detection wavelength is 300nm; the column temperature is 34°C; the number of theoretical plates should not be less than 2000 based on the peak of schisandrin A;
[0067] (2) Preparation of reference substance solution: take an appropriate amount of schisandrin A reference substance, weigh it accurately, add methanol to make a solution containing 0.3 mg of schisandrin A per 1 mL, and obtain it;
[0068] (3) Preparation of the test solution: take 20g of Yixinshu Granules, mix well; take 1g, accurately weigh it, put it in a 25mL volumetric flask, add 20mL of methanol, and ultrasonicate for 20min; take it out, let it cool, and add methanol to the scale, shake well, filter, and take the filtrate to ...
Embodiment 3
[0071] Determination of Schisandrin A in Yixinshu Tablets (commercially available):
[0072] (1) Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; methanol: water = 70:30 is used as mobile phase; flow rate is 0.5mL min -1 ; The detection wavelength is 200nm; the column temperature is 28℃;
[0073] (2) Preparation of reference substance solution: Accurately weigh 3.22 mg of schisandrin A reference substance, place it in a 10 mL volumetric flask, add methanol to dissolve and set the volume to the mark to make a solution with a concentration of 0.322 mg / mL, and pass through 0.22 μm The microporous membrane, take the continued filtrate, to obtain final product;
[0074] (3) Preparation of the test solution: Take 15 tablets of Yixinshu and mix them evenly; take 1g, accurately weigh it, put it in a 25mL volumetric flask, add 20mL of methanol, and ultrasonicate for 15min; take it out, let it cool, add Methanol to the mark, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com