Patents
Literature
313 results about "Transposon element" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A transposable element (TE or transposon) is a DNA sequence that can change its position within a genome, sometimes creating or reversing mutations and altering the cell's genetic identity and genome size.
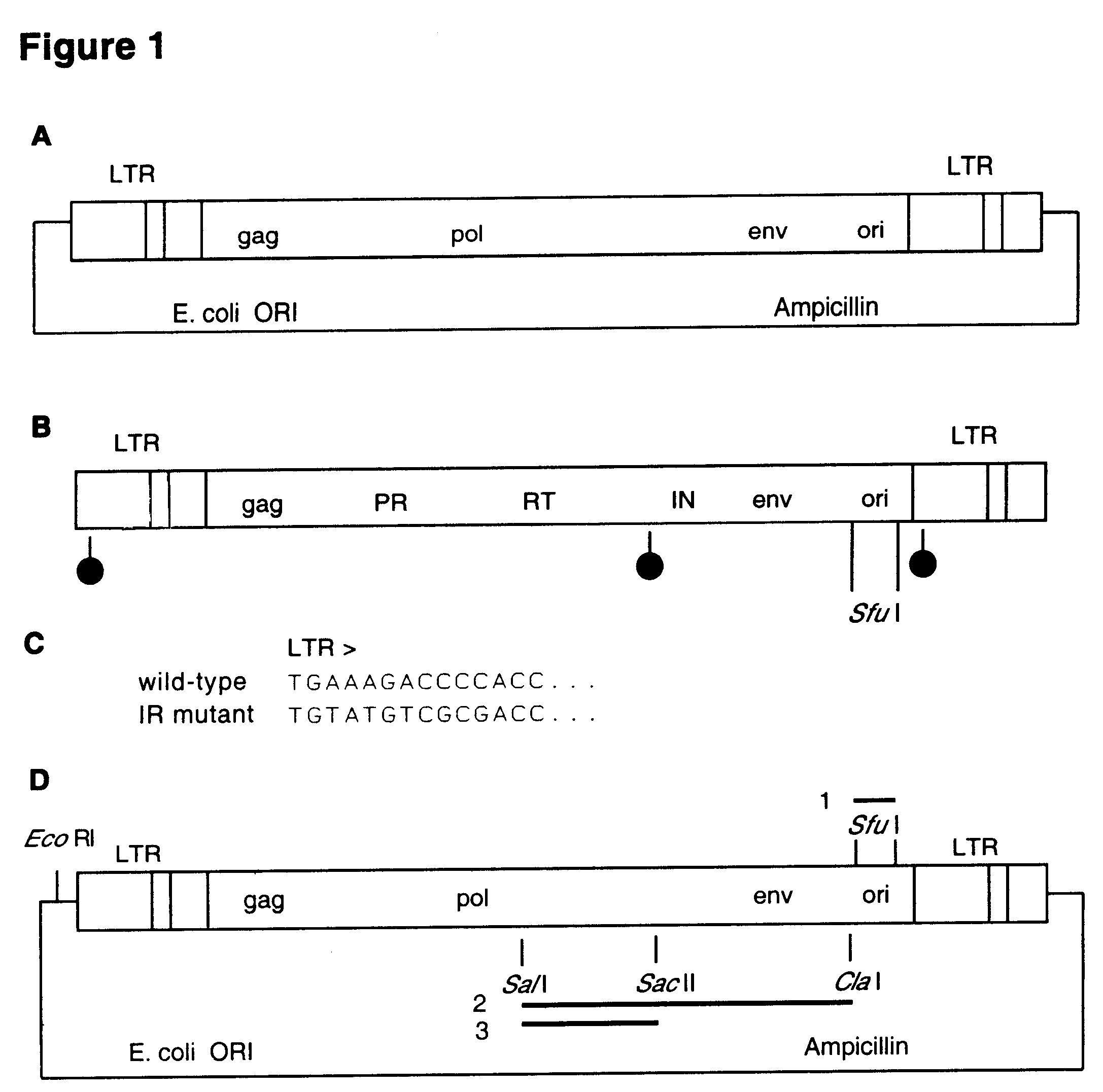
Transposon end compositions and methods for modifying nucleic acids
ActiveUS20100120098A1Sugar derivativesMicrobiological testing/measurementGenomic sequencingPolymerase L
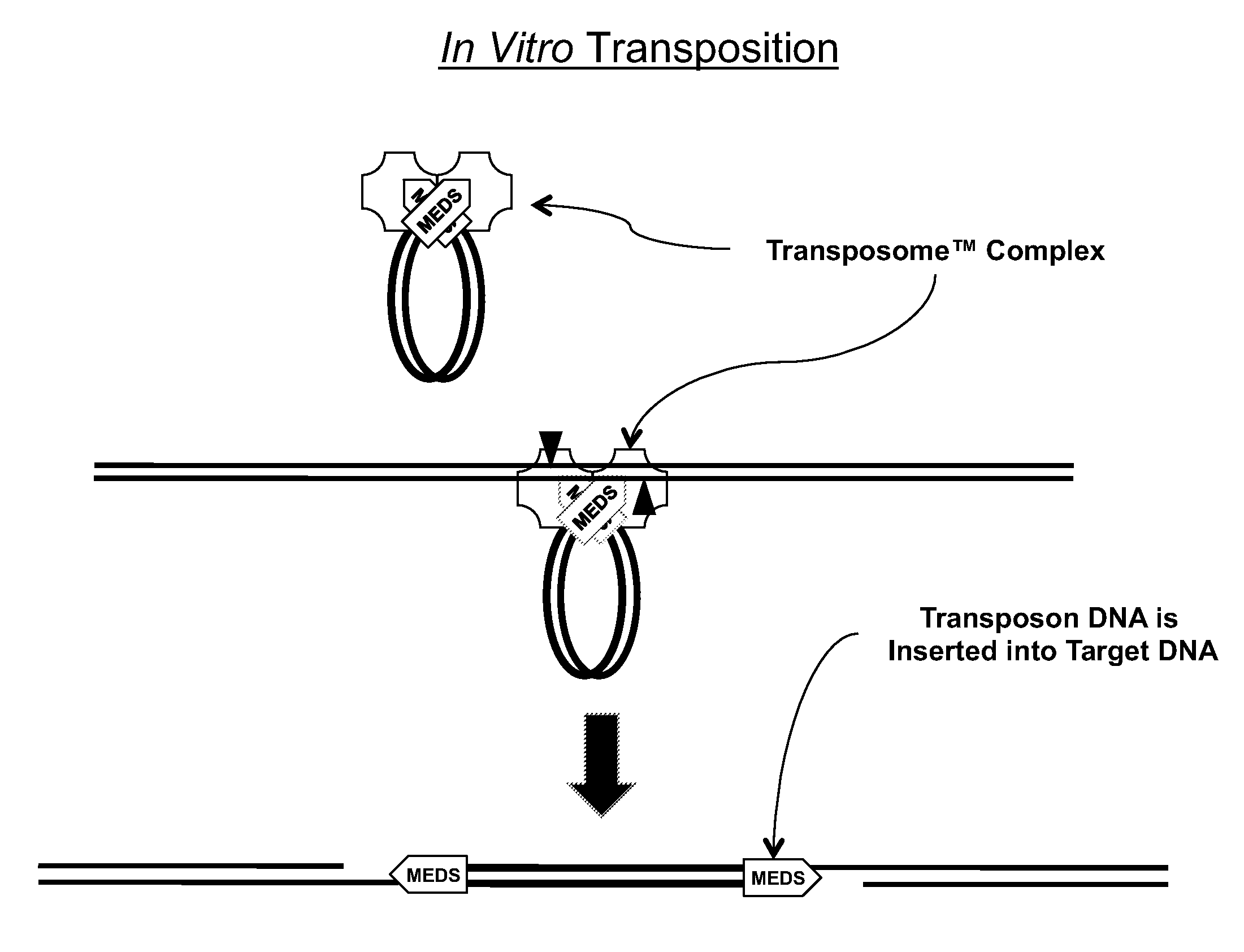
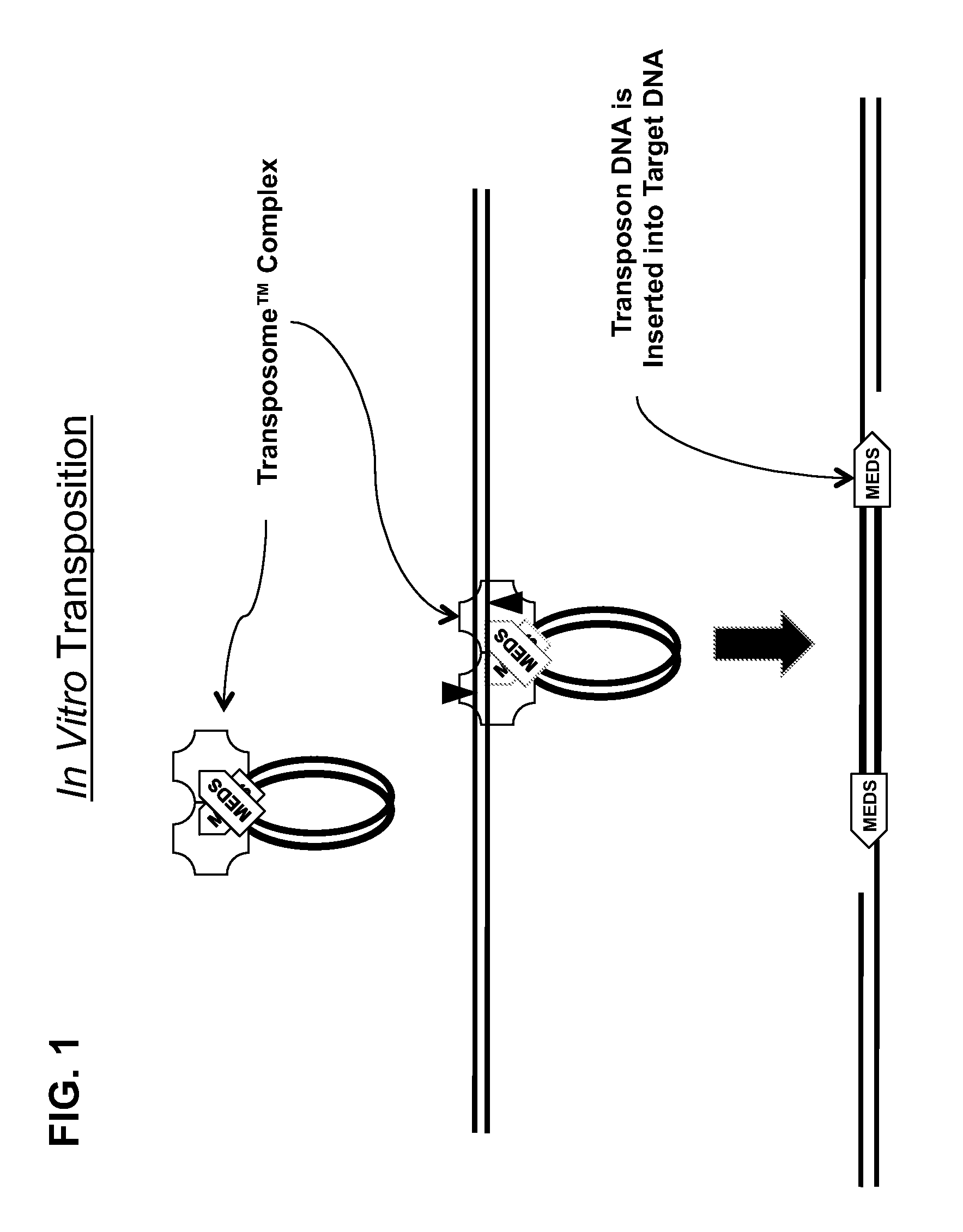
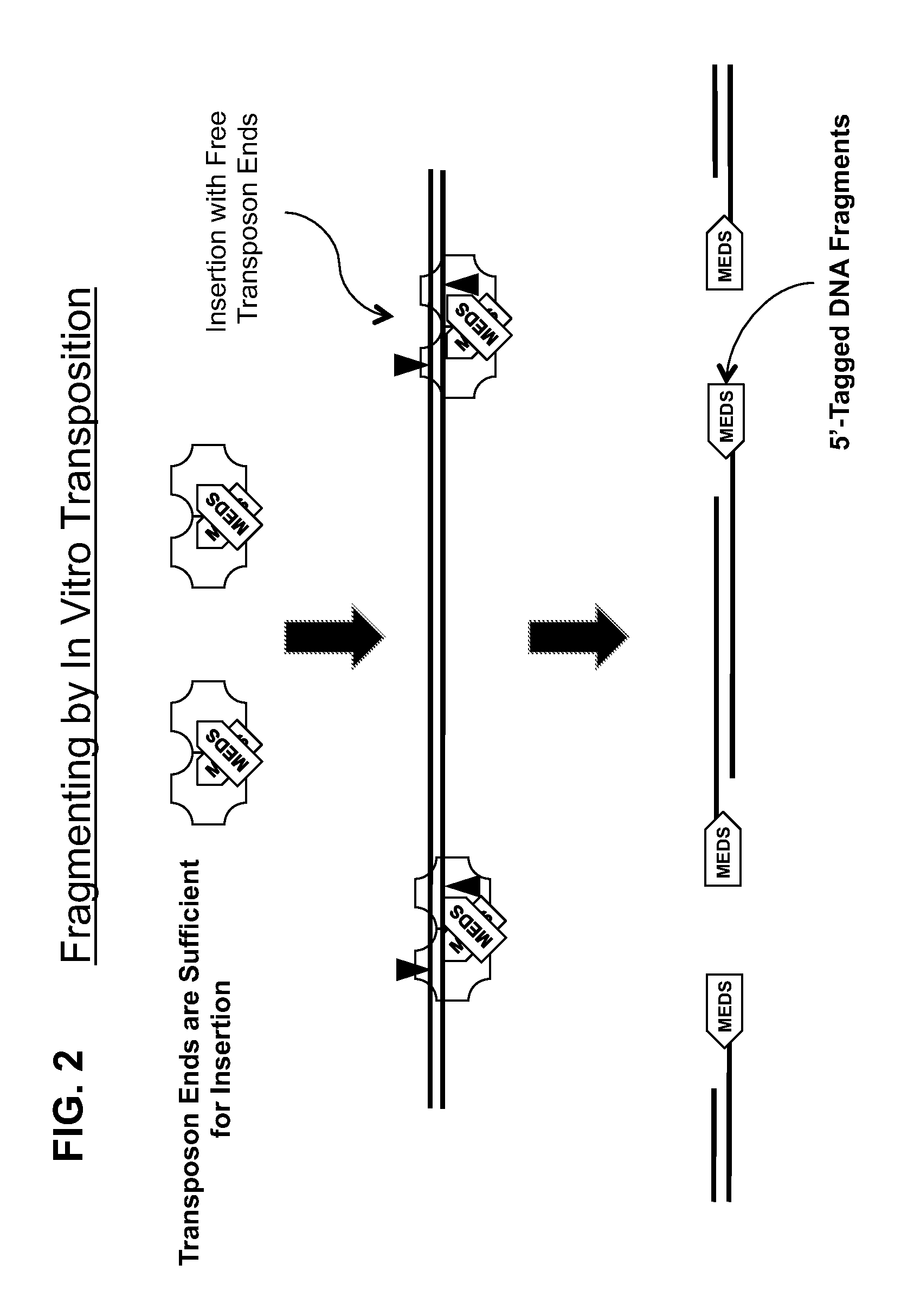
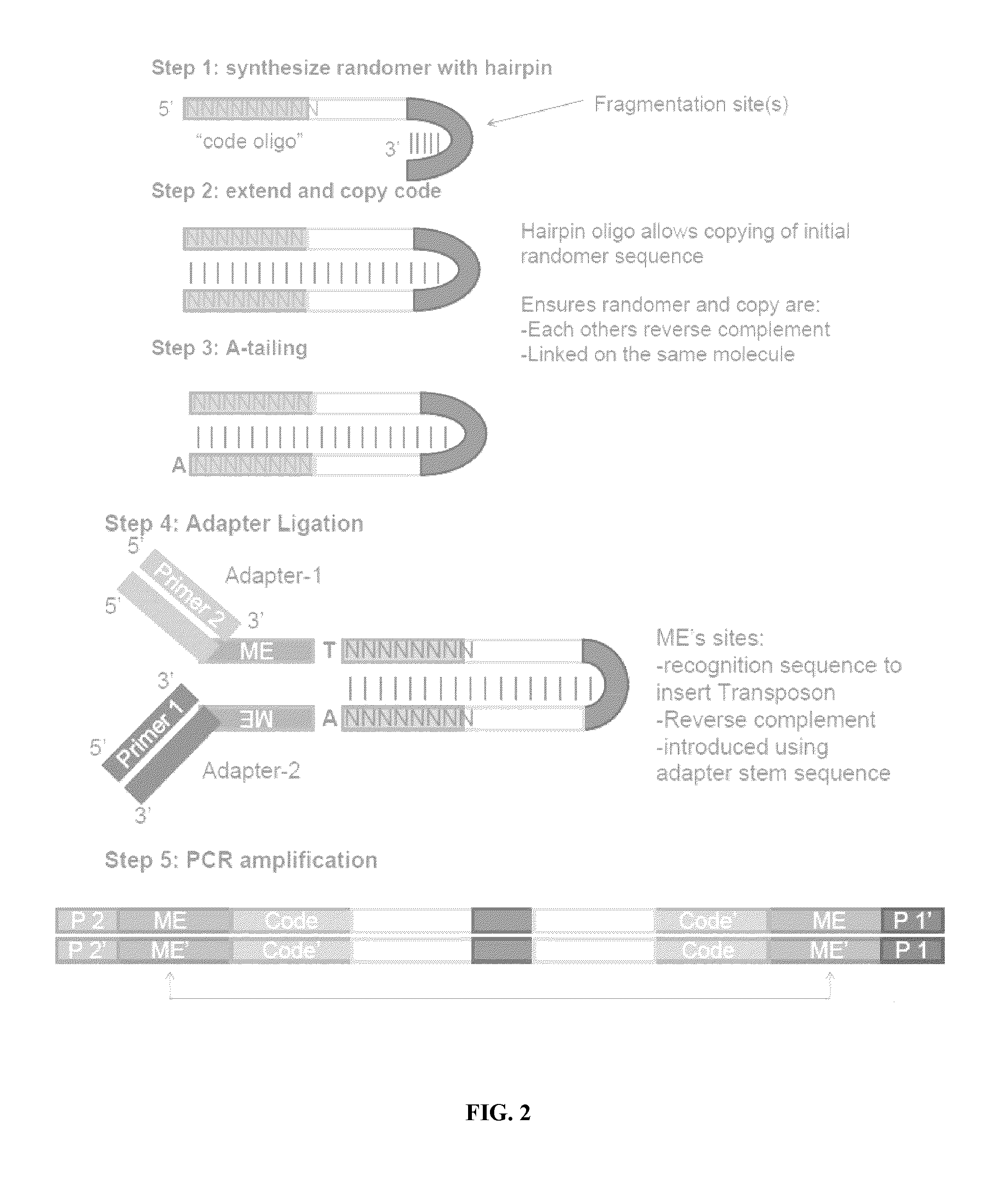
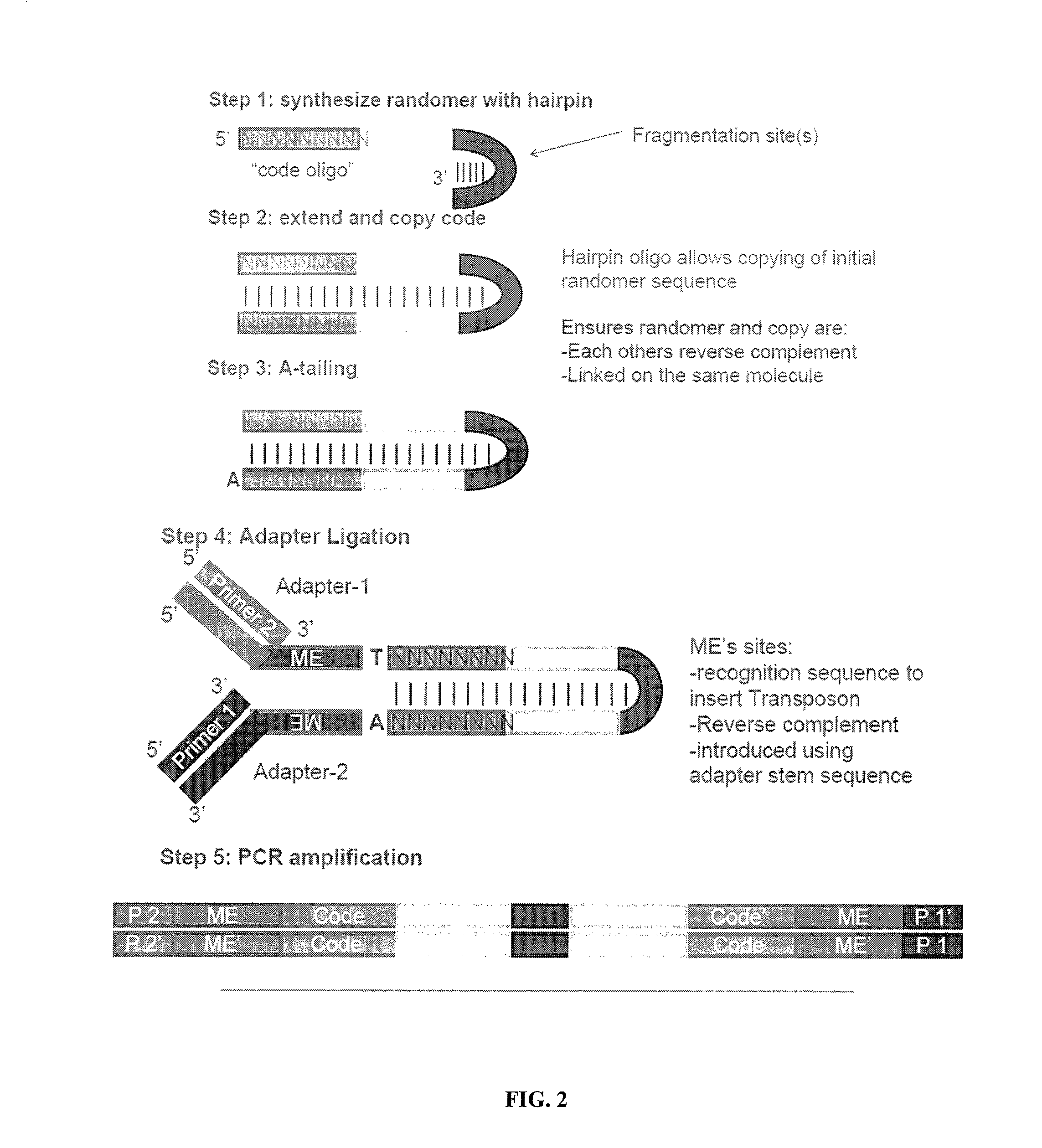
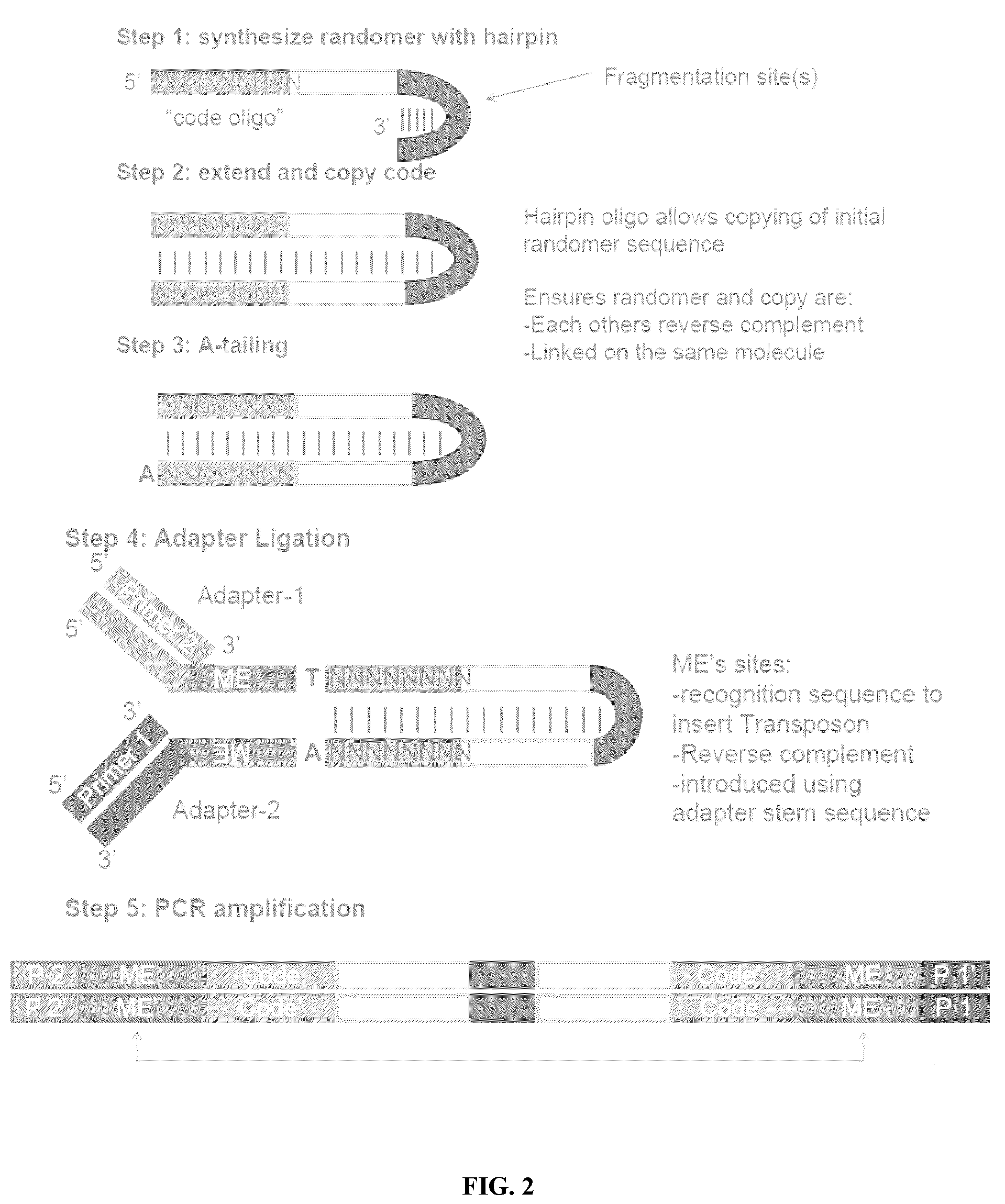
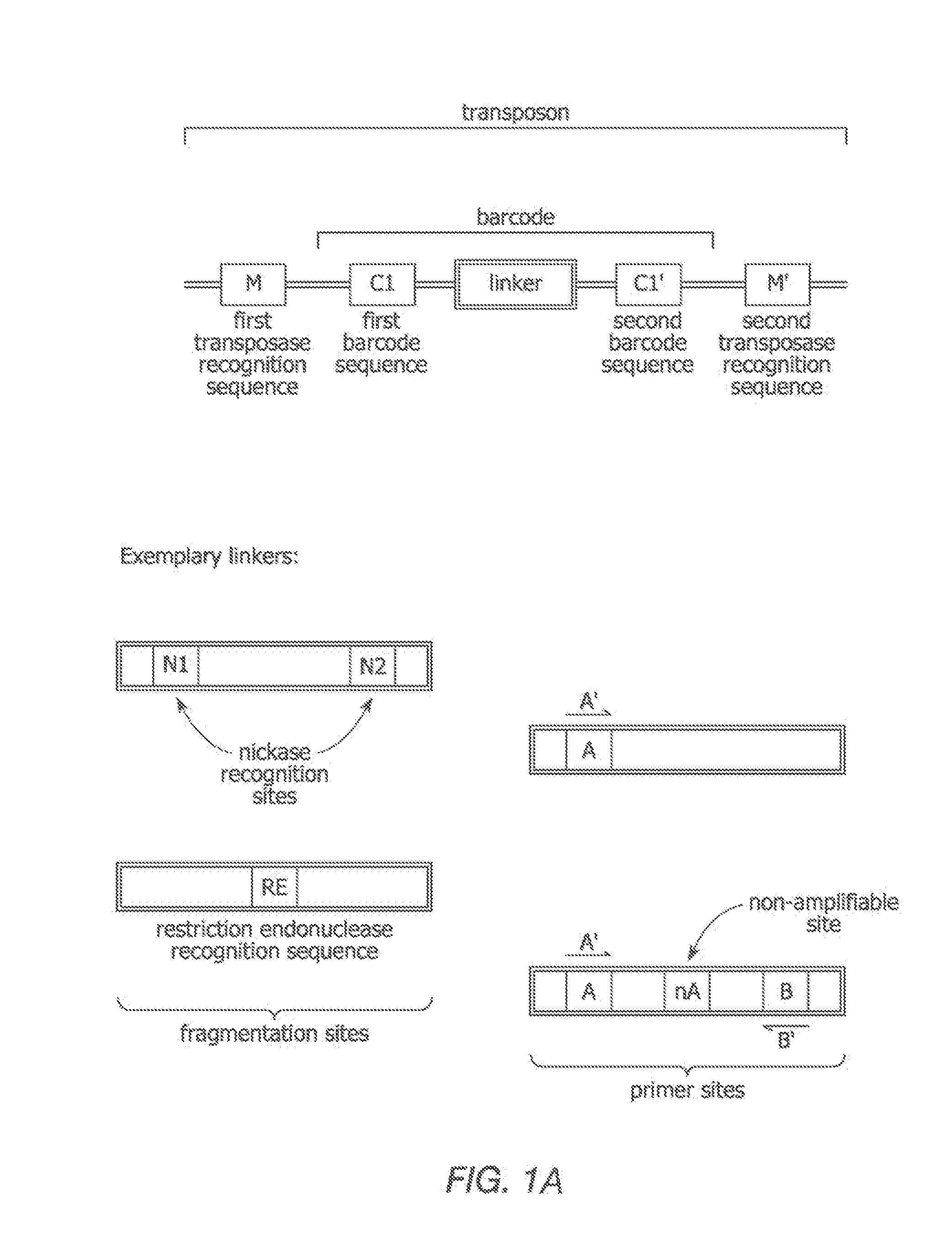
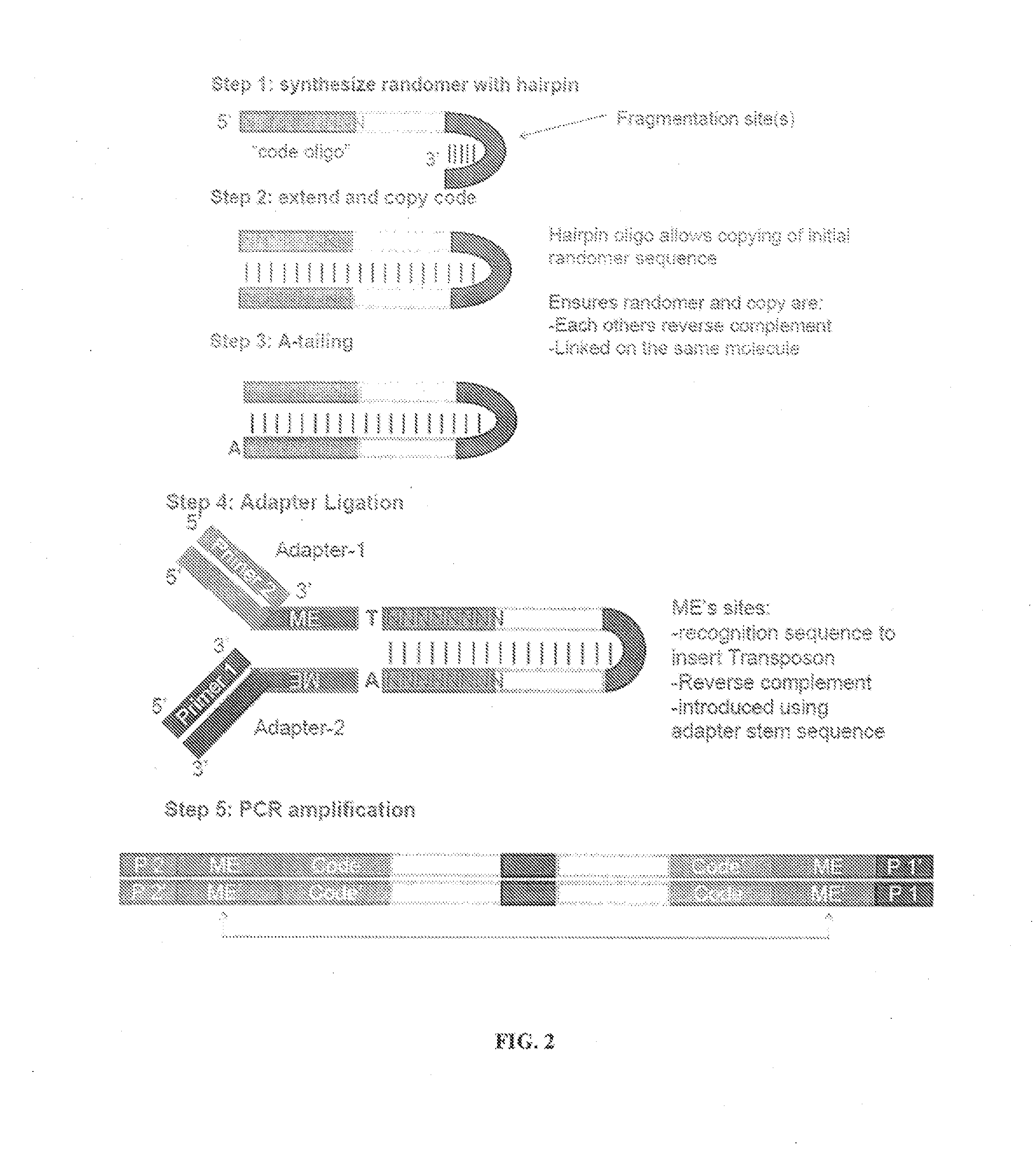
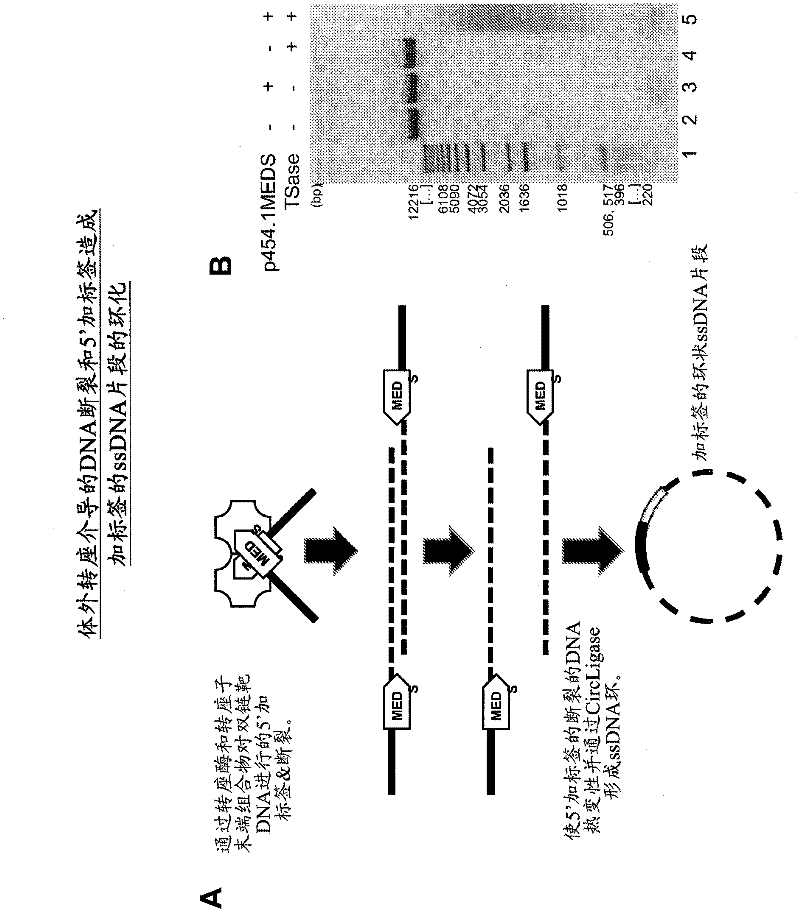
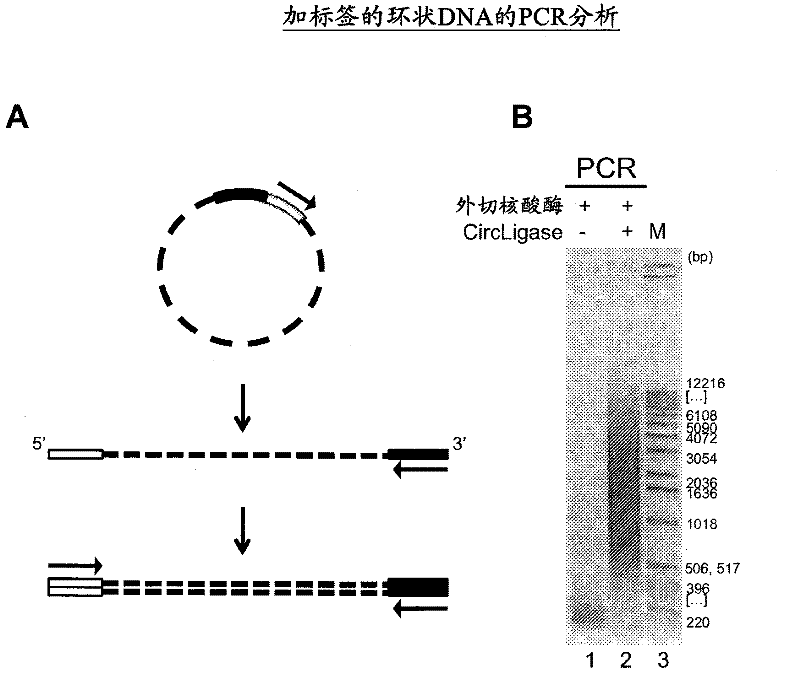
The present invention provides methods, compositions and kits for using a transposase and a transposon end for generating extensive fragmentation and 5′-tagging of double-stranded target DNA in vitro, then using a DNA polymerase for generating 5′- and 3′-tagged single-stranded DNA fragments without performing a PCR amplification reaction, wherein the first tag on the 5′-ends exhibits the sequence of the transferred transposon end and optionally, an additional arbitrary sequence, and the second tag on the 3′-ends exhibits a different sequence from the sequence exhibited by the first tag. The method is useful for generating 5′- and 3′-tagged DNA fragments for use in a variety of processes, including processes for metagenomic analysis of DNA in environmental samples, copy number variation (CNV) analysis of DNA, and comparative genomic sequencing (CGS), including massively parallel DNA sequencing (so-called “next-generation sequencing.)
Owner:ILLUMINA INC
Linking sequence reads using paired code tags
ActiveUS20120208724A1Reduce in quantitySugar derivativesMicrobiological testing/measurementComputational biologyTransposon element
Owner:ILLUMINA INC
Linking sequence reads using paired code tags
ActiveUS20120208705A1Reduce in quantitySugar derivativesMicrobiological testing/measurementComputational biologyTransposon element
Owner:ILLUMINA INC
Methods of in vivo gene transfer using a sleeping beauty transposon system
InactiveUS6613752B2Observed effectPromote cloningBiocideHydrolasesSleeping Beauty transposon systemMulticellular organism
Methods and compositions for introducing a nucleic acid into the genome of at least one cell of a multicellular organism are provided. In the subject methods, a Sleeping Beauty transposon that includes the nucleic acid is administered to the multicellular organism along with a source of a Sleeping Beauty transposase activity. Administration of the transposon and transposase results in integration of the transposon, as well as the nucleic acid present therein, into the genome of at least one cell of the multicellular organism The subject methods find use in a variety of different applications, including the in vivo transfer of genes for use in, among other applications, gene therapy applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Retrovirus and viral vectors
InactiveUS6635472B1Prevent and attenuate diseaseEnhance immune responseGenetic material ingredientsVirus peptidesVirus-RetrovirusDna viral
This invention relates to the fields of genetic engineering, virus replication and gene transfer. More specifically, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors, wherein an ori derived from a DNA virus capable of replicating in vertebrate cells is inserted into the retrovirus, allowing the retrovirus following the reverse transcription to efficiently replicate as extrachromosomal or episomal DNA without the necessity of integration into the host cell chromosome. Additionally, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors replicating episomally without aid of an ori and related elements. Also, this invention encompasses preventive, therapeutic, and diagnostic applications employing said constructs, viruses and vectors.
Owner:RUBICON LABS
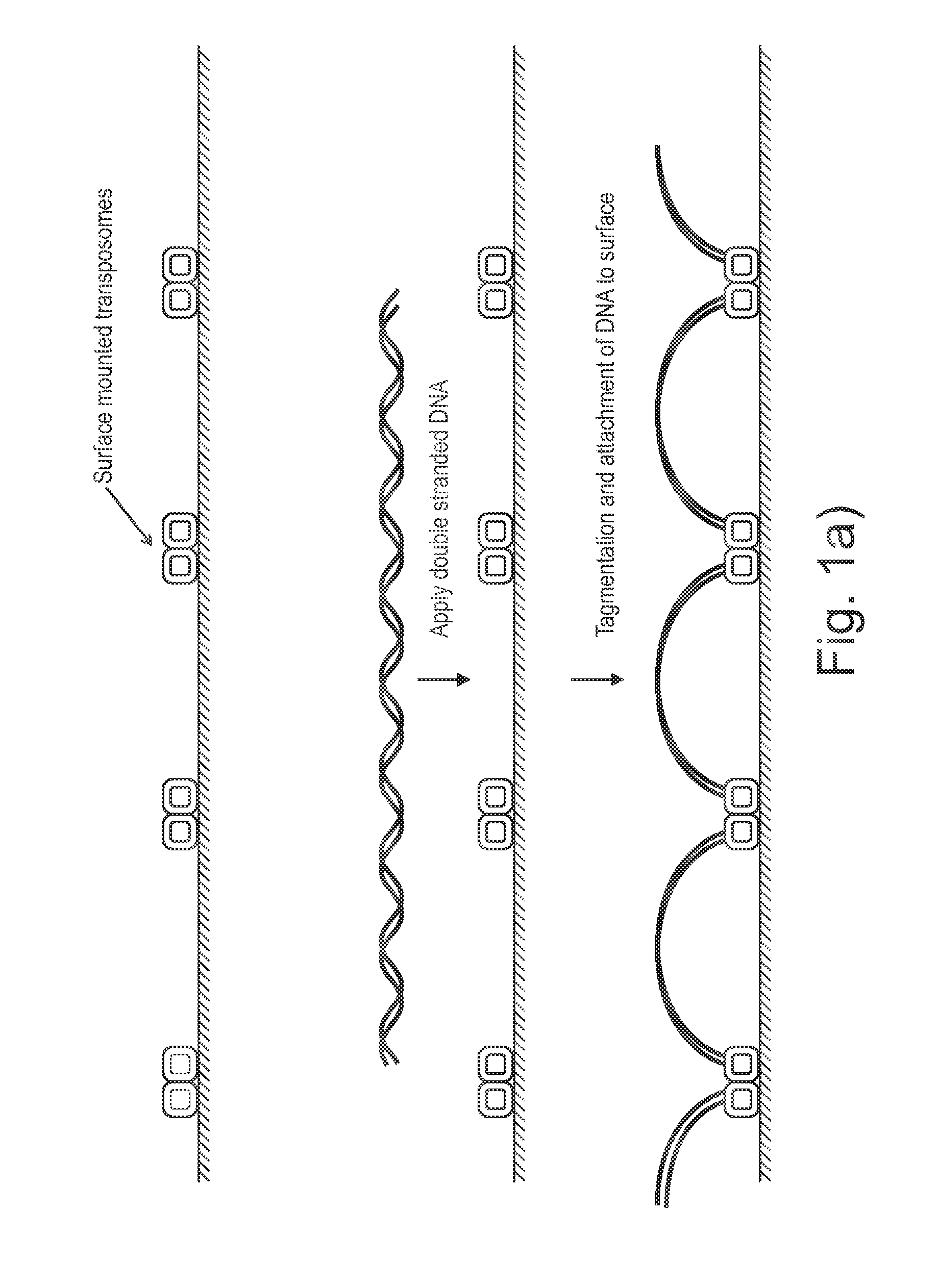
Sample preparation on a solid support
ActiveUS20140194324A1Sequential/parallel process reactionsMicrobiological testing/measurementSolid massDNA fragmentation
Presented are methods and compositions for using immobilized transposase and a transposon end for generating an immobilized library of 5′-tagged double-stranded target DNA on a surface. The methods are useful for generating 5′- and 3′-tagged DNA fragments for use in a variety of processes, including massively parallel DNA sequencing.
Owner:ILLUMINA CAMBRIDGE LTD
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
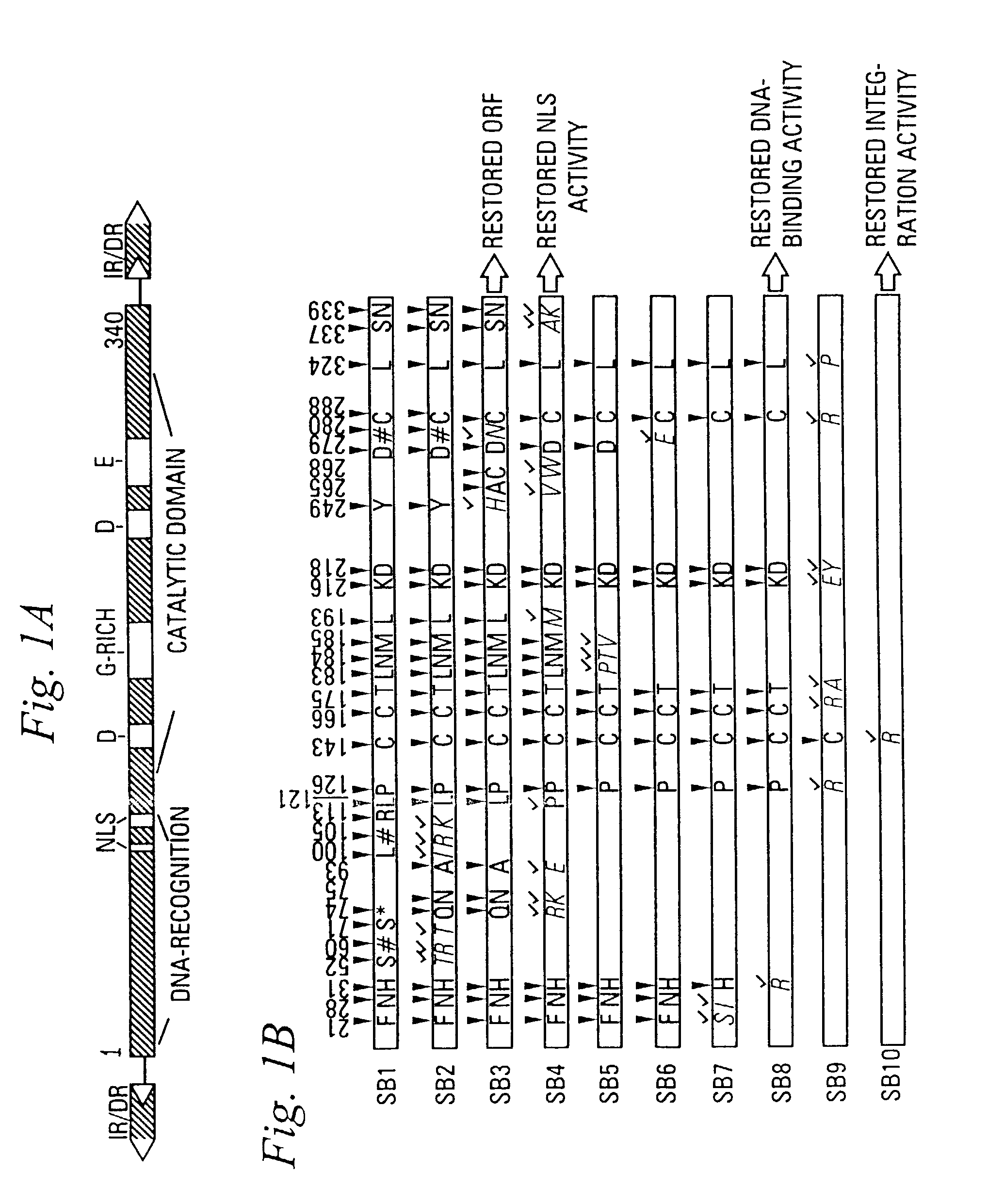
Enhanced sleeping beauty transposon system and methods for using the same
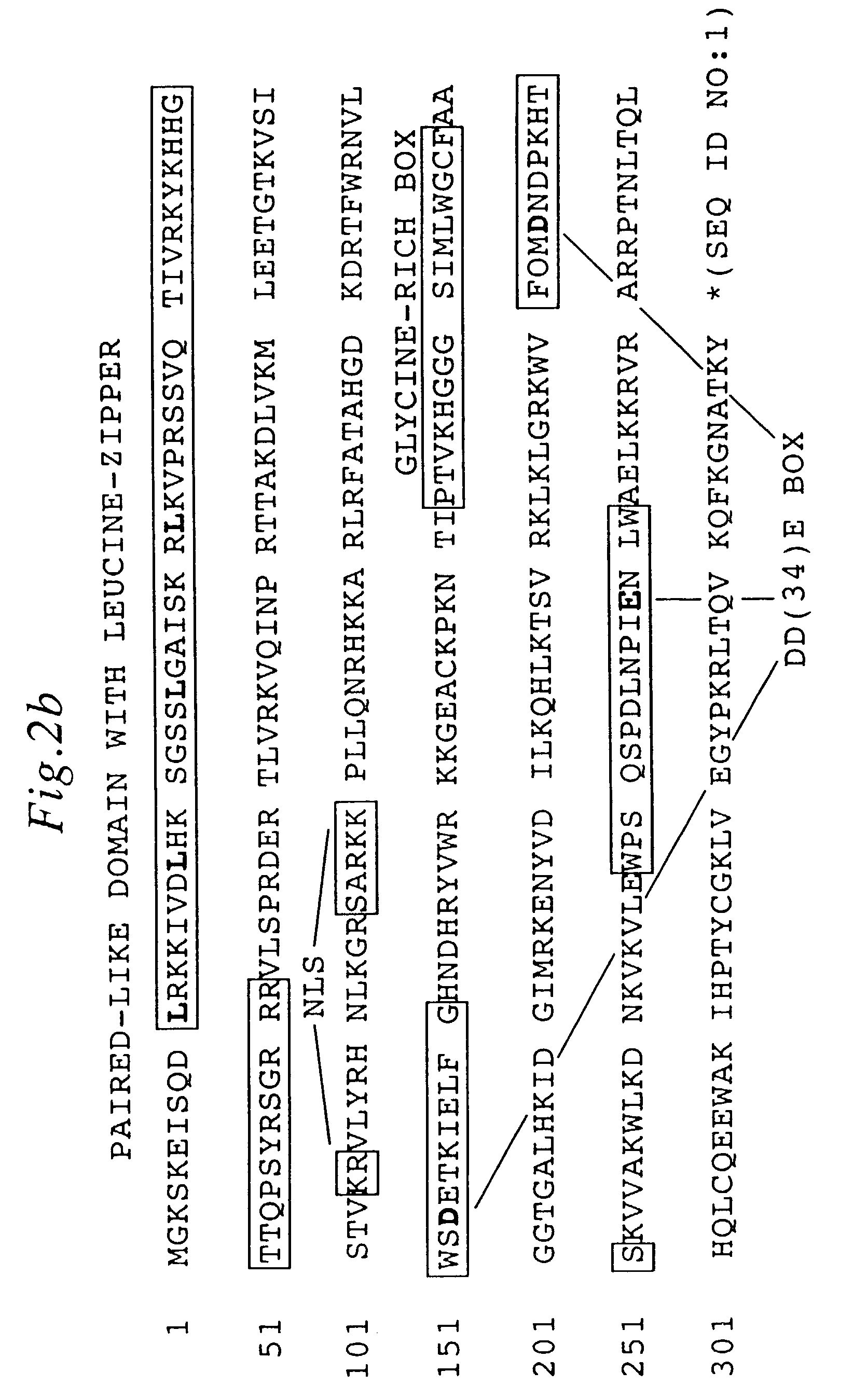
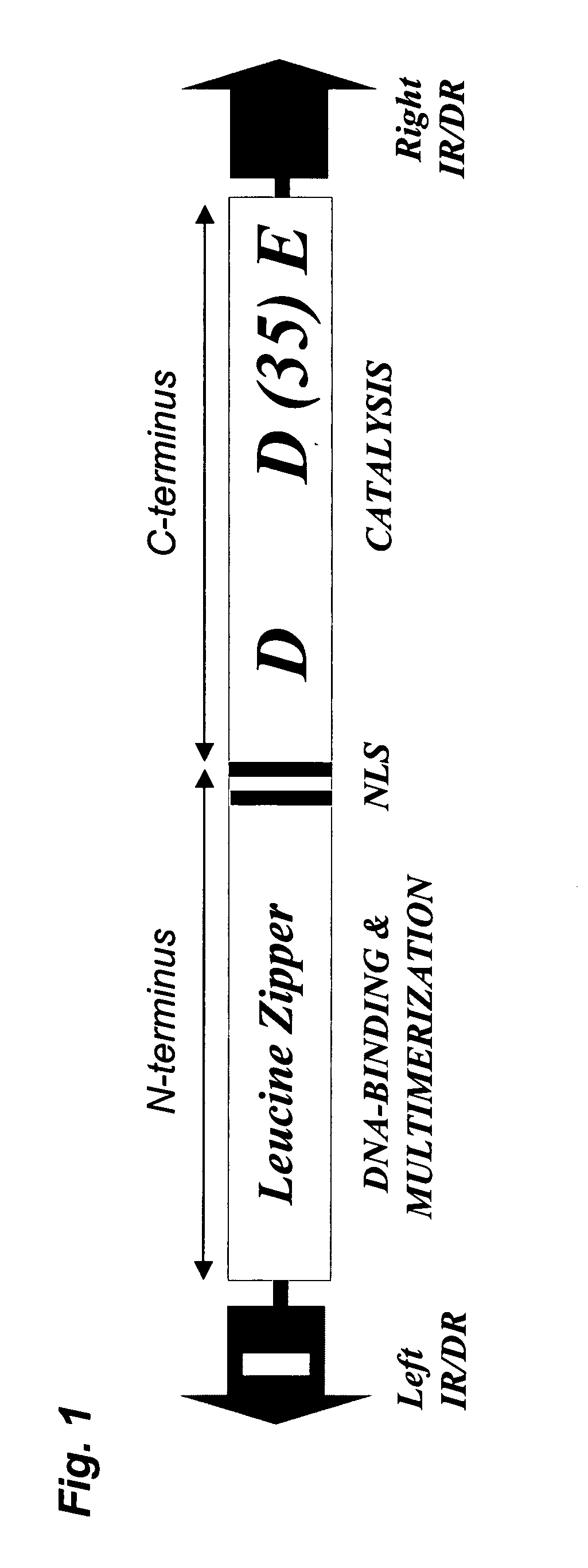
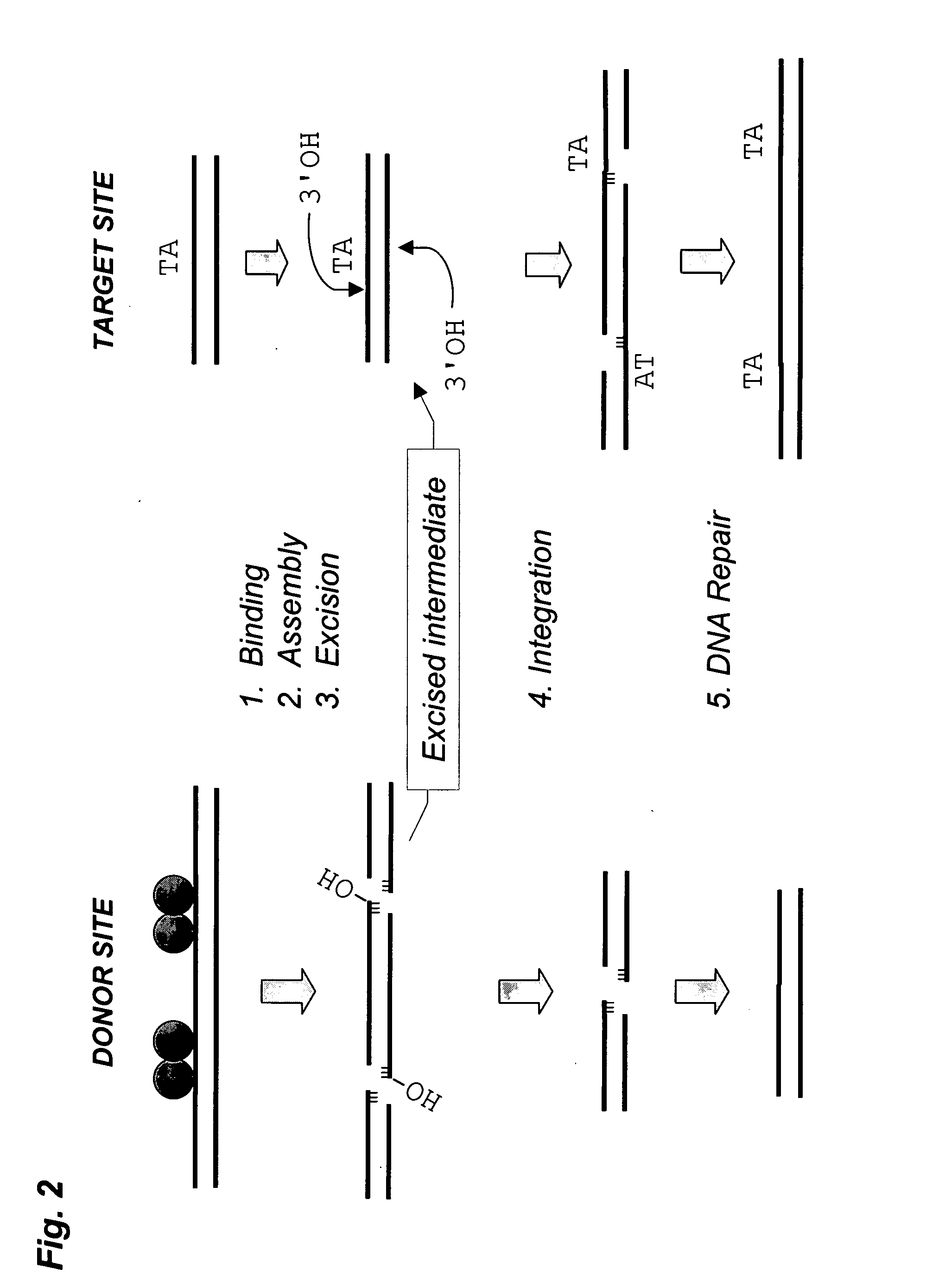
Methods and compositions for introducing a nucleic acid into the genome of a cell are provided. In the subject methods, a Sleeping Beauty transposon that includes the nucleic acid is introduced into the cell along with a source of a mutant Sleeping Beauty transposase that provides for enhanced integration as compared to the wild-type Sleeping Beauty transposase having an amino acid sequence as shown in SEQ ID NO:01. Introduction of the mutant Sleeping Beauty Transposase and transposon results in integration of the nucleic acid into the cell genome. Also provided are mutant transposases and transposons, as well as systems and kits thereof, that find use in practicing the subject methods. The subject methods and compositions find use in a variety of different applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Nucleic acid transfer vector for the introduction of nucleic acid into the DNA of a cell
Owner:RGT UNIV OF MINNESOTA
Methods and compositions for amplifying DNA clone copy number
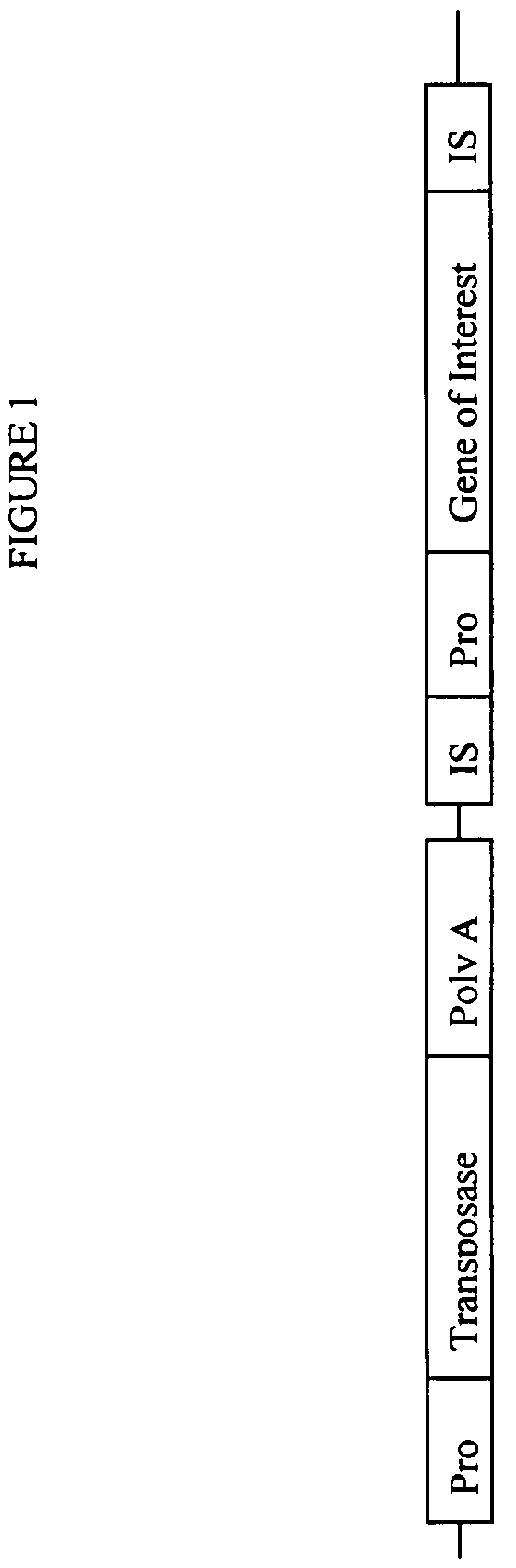
A method for retrofitting DNA in a single-copy or high-copy vector, such as a fosmid or BAC, whereby an artificial transposon is used to introduce a conditional multi-copy origin of replication (“ori”) into the DNA in said vector. Following random in vitro or in vivo transposition of the ori-containing transposon into DNA in the single-copy or low-copy vector, the resulting insertion clones are introduced into a special host strain that contains a gene which encodes a polypeptide required for replication from the multi-copy ori. However, since the gene for this polypeptide is expressed from a tightly-regulated inducible promoter, the polypeptide is not expressed in the absence of inducer. On addition of inducer to the culture medium, the host cell synthesizes the polypeptide, which in turn activates replication from the multi-copy ori, thereby increasing the amount of clone DNA synthesized by the cell.
Owner:EPICENT TECH CORP
Linking sequence reads using paired code tags
ActiveUS9074251B2Reduce in quantityMicrobiological testing/measurementDNA preparationComputational biologyTransposon element
Owner:ILLUMINA INC
Linking sequence reads using paired code tags
ActiveUS8829171B2Reduce in quantitySugar derivativesMicrobiological testing/measurementComputational biologyTransposon element
Owner:ILLUMINA INC
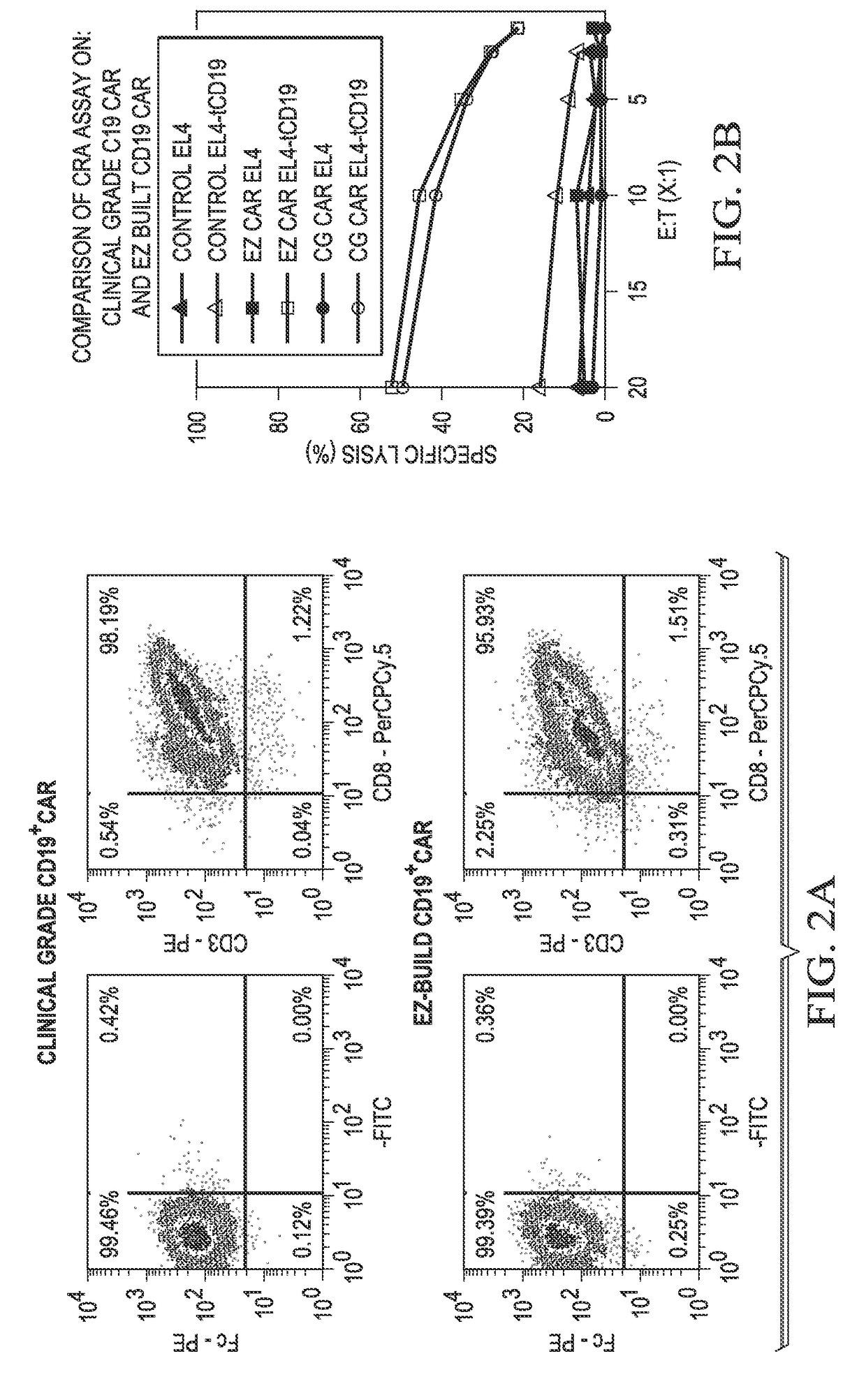
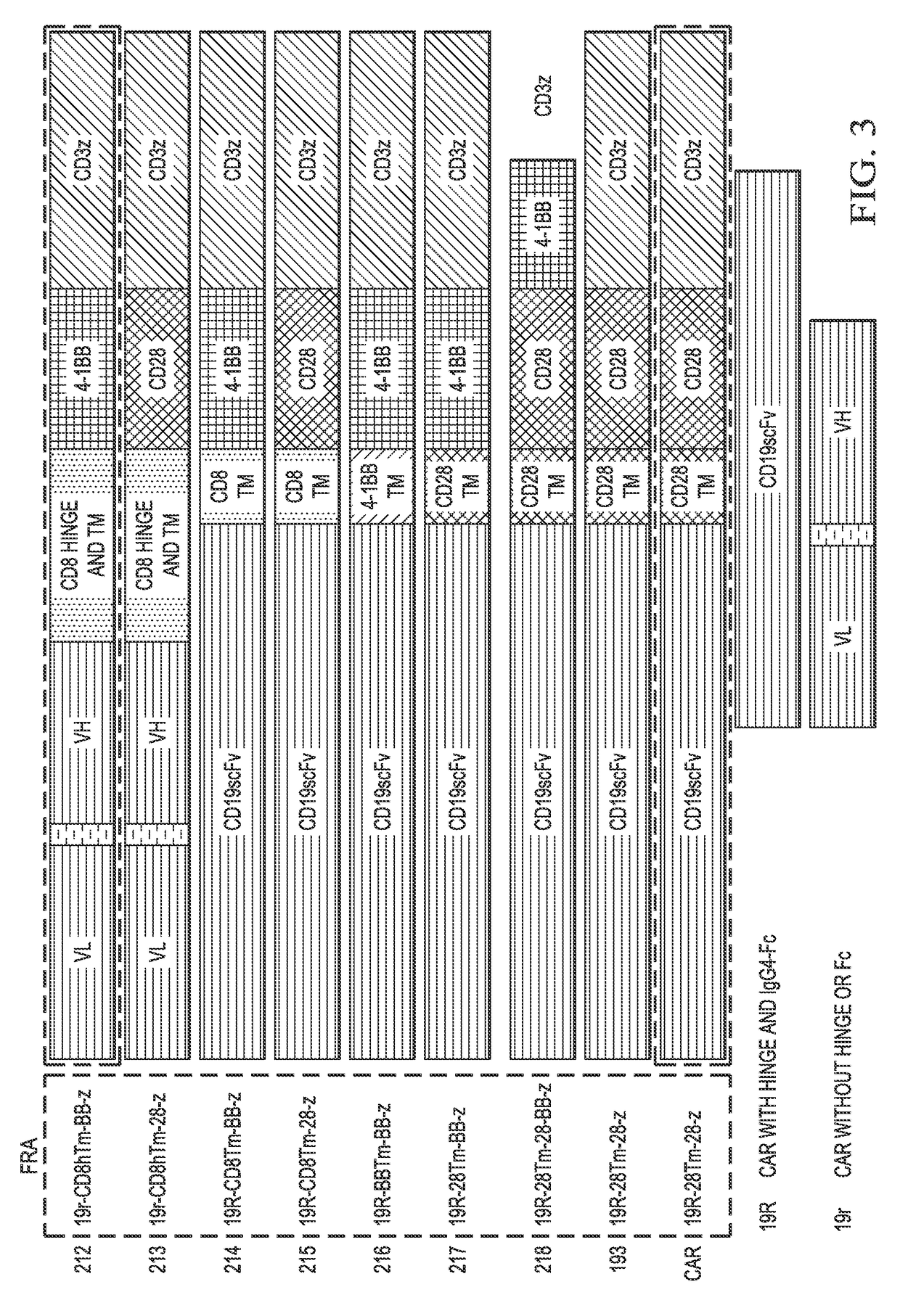
Human application of engineered chimeric antigen receptor (CAR) T-cells
ActiveUS9629877B2Promote proliferation and survivalInhibit expressionImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsHuman bodyElectroporation
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising a chimeric antigen receptor (CAR). In particular aspects, CAR-expressing T-cells are producing using electroporation in conjunction with a transposon-based integration system to produce a population of CAR-expressing cells that require minimal ex vivo expansion or that can be directly administered to patients for disease (e.g., cancer) treatment.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Haploidome determination by digitized transposons
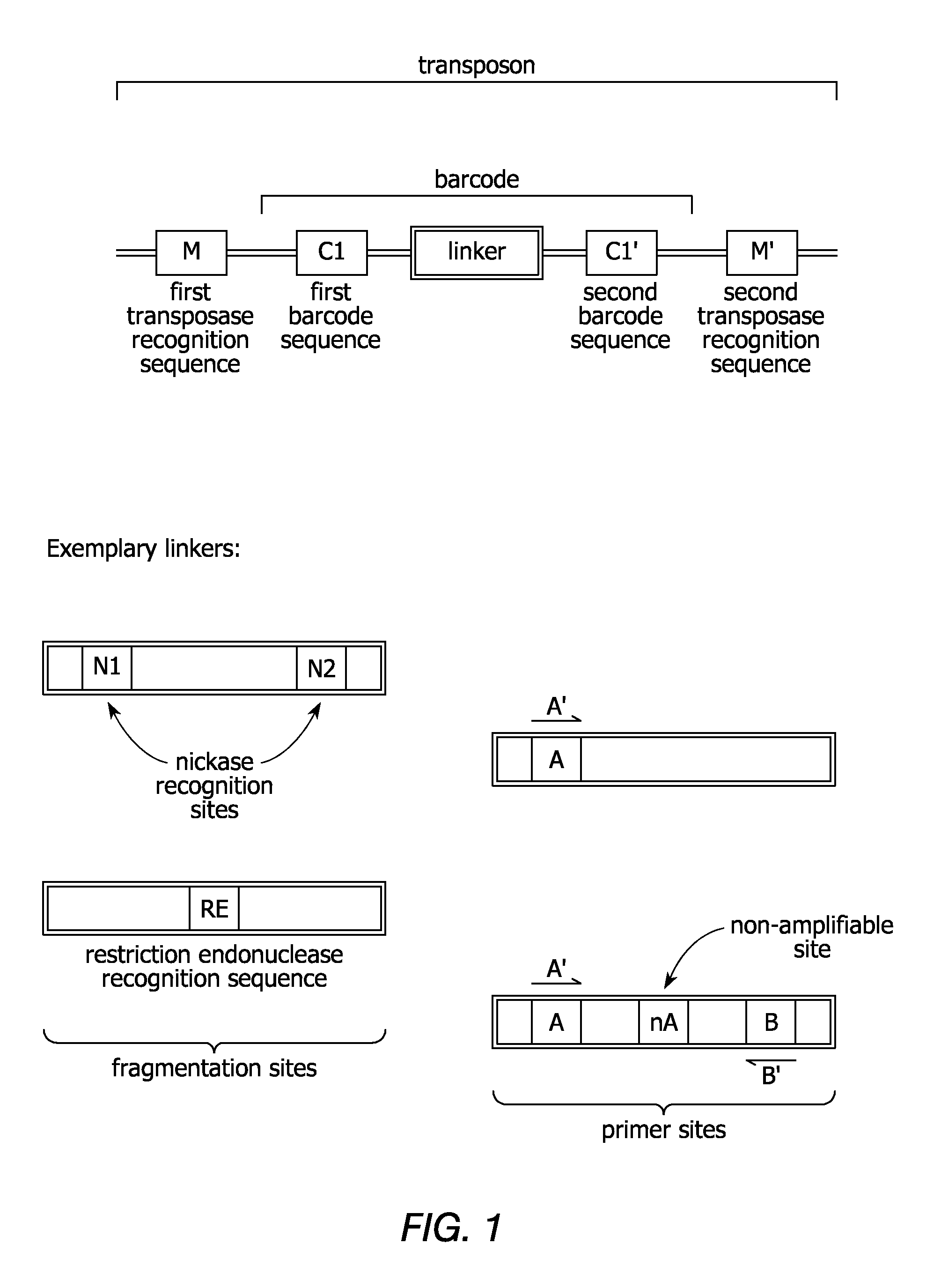
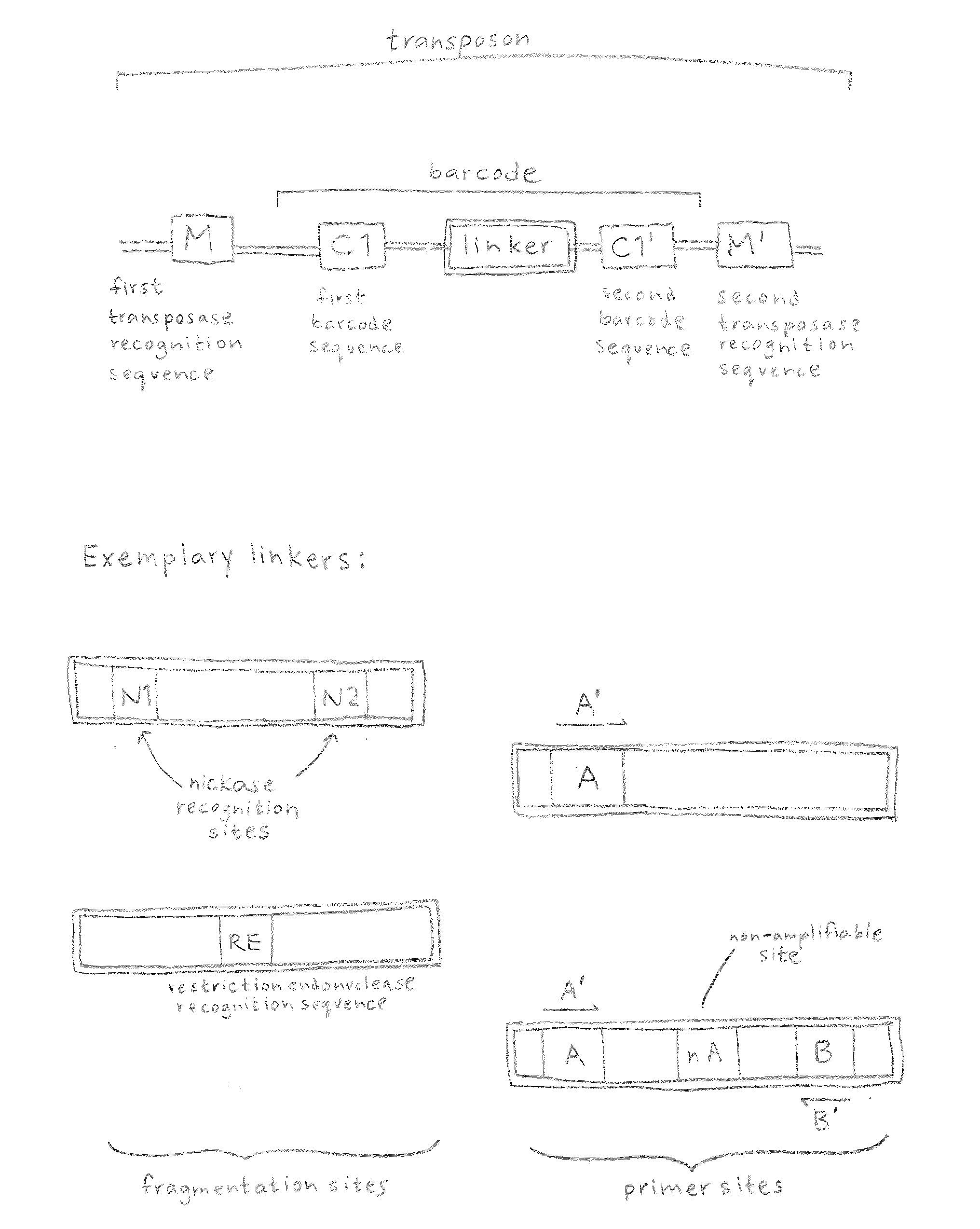
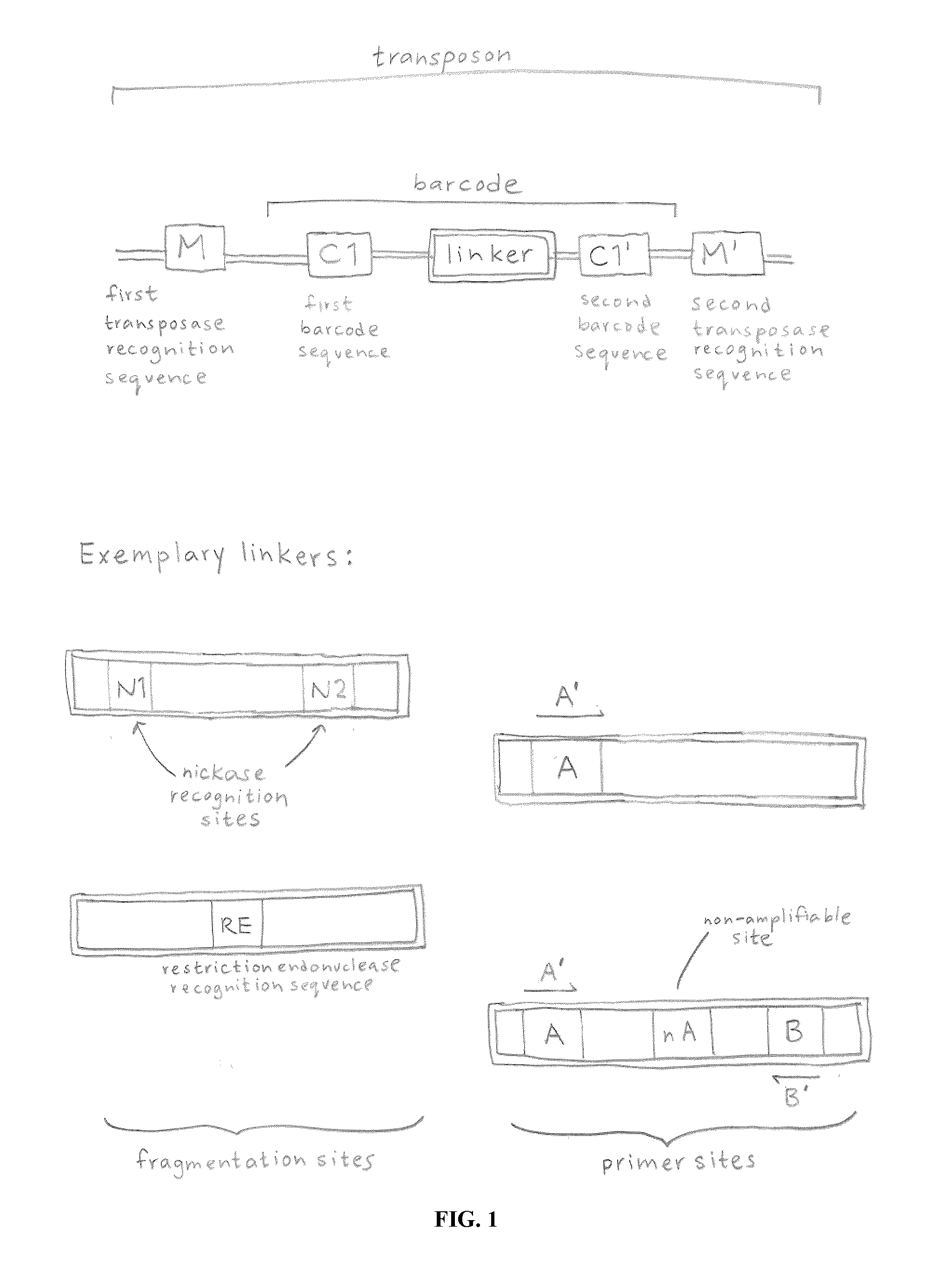
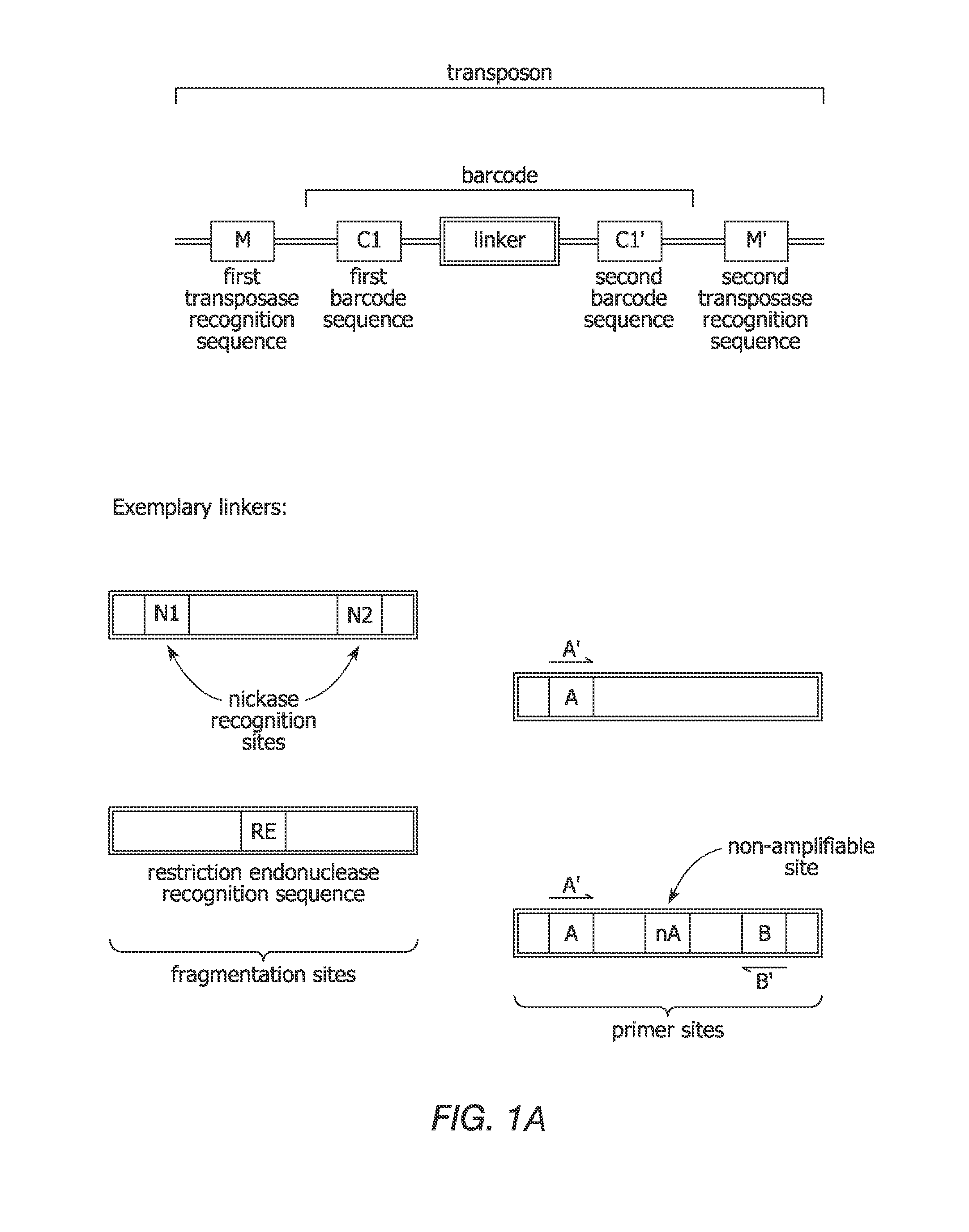
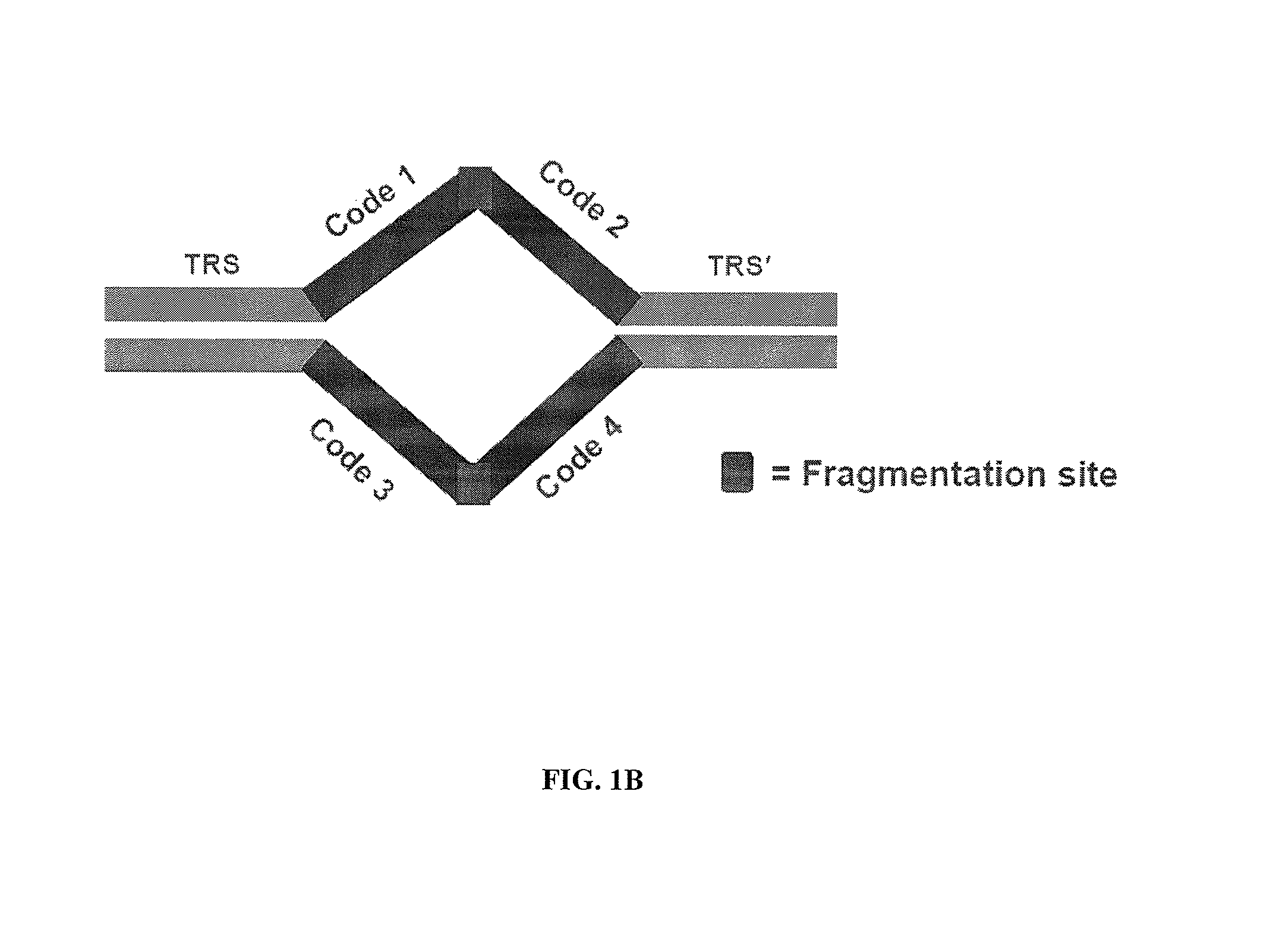
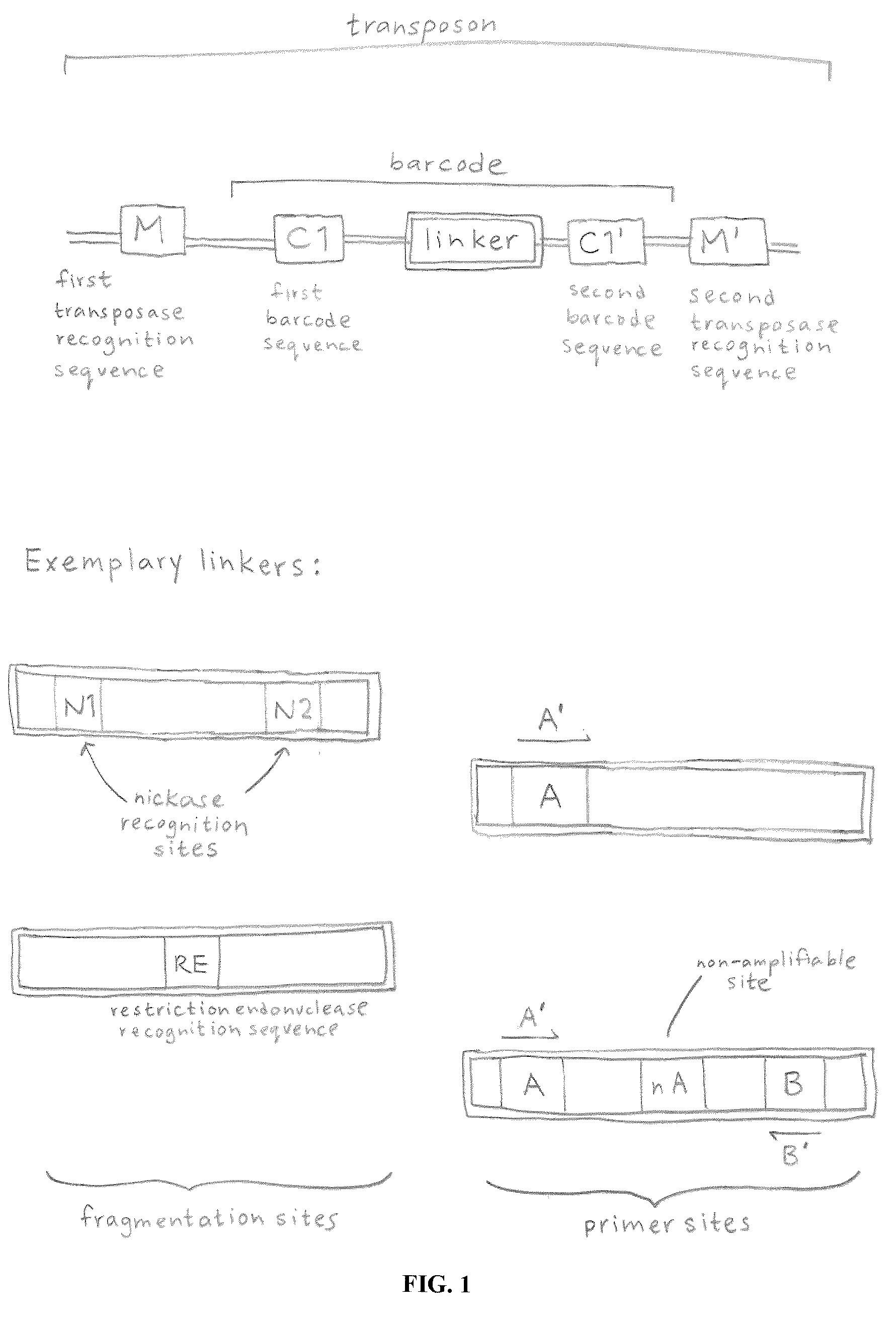
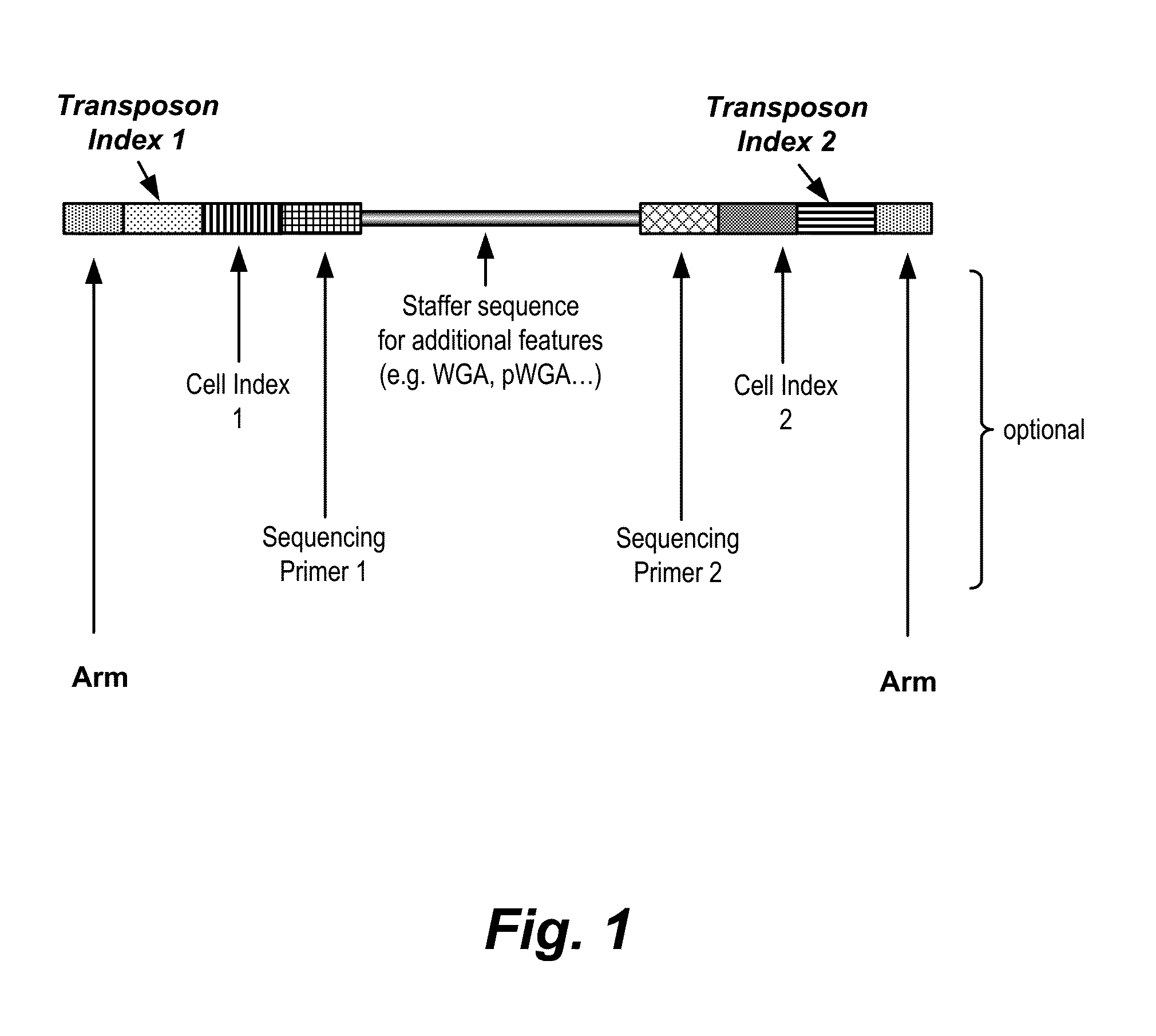
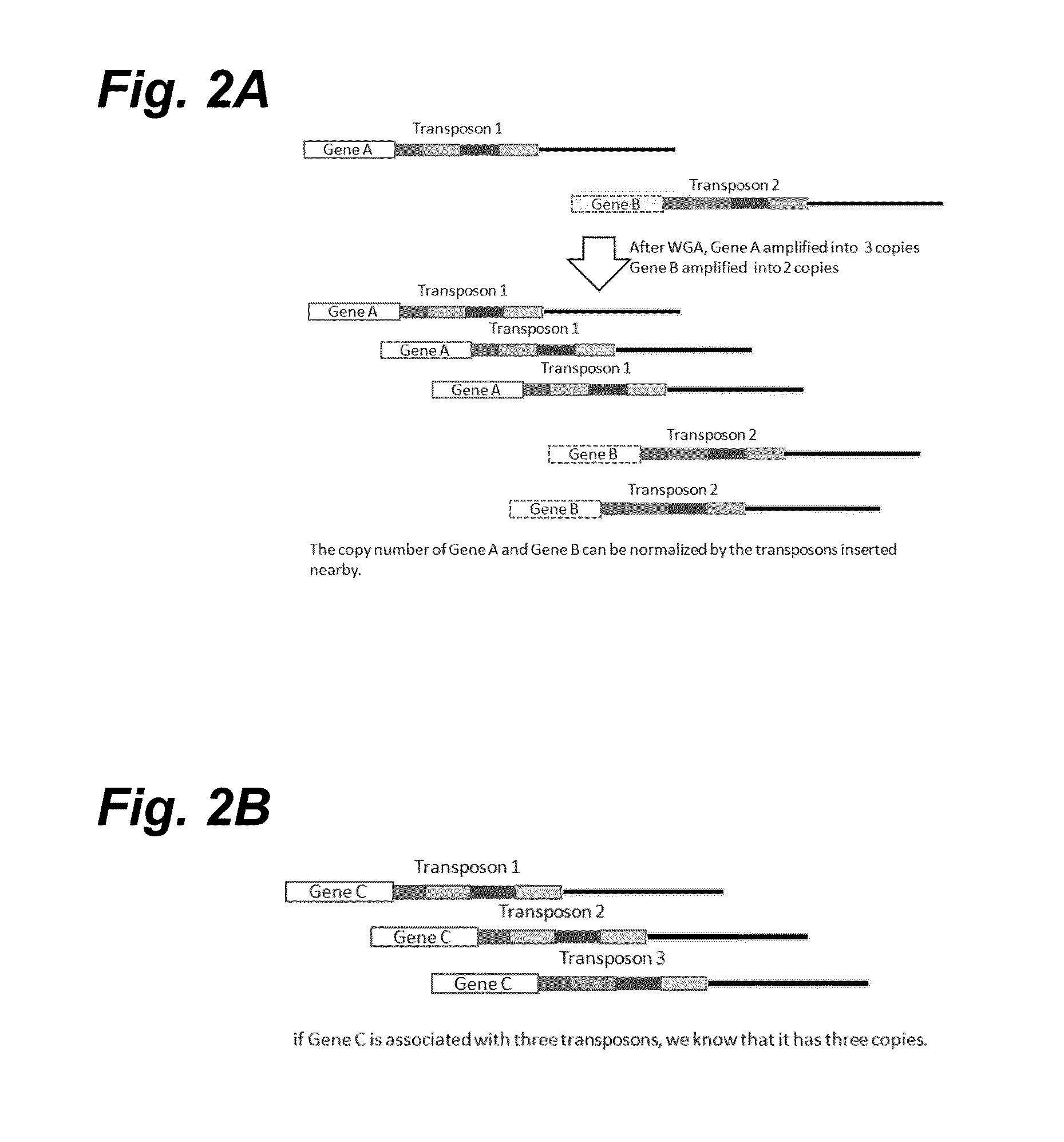
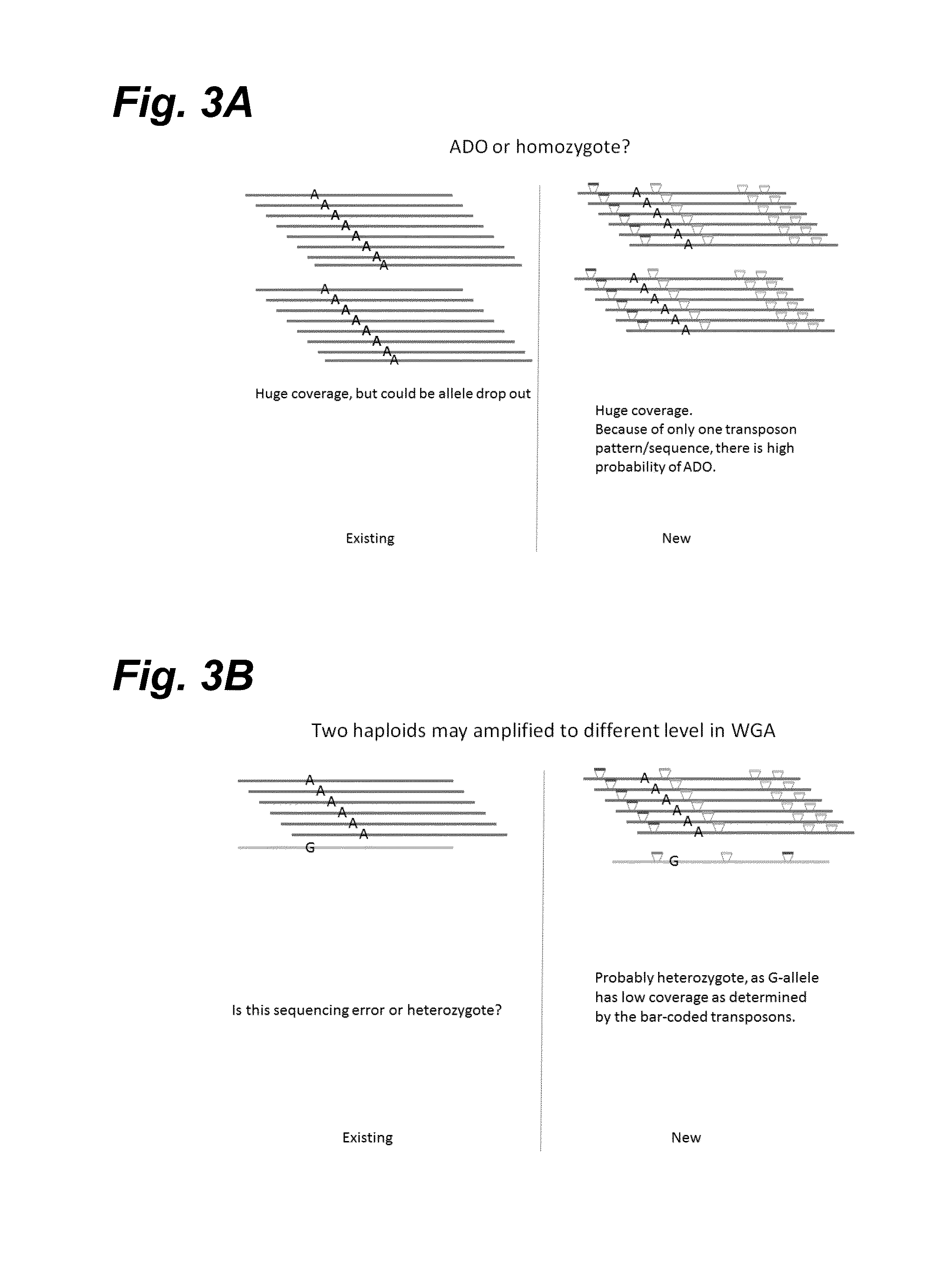
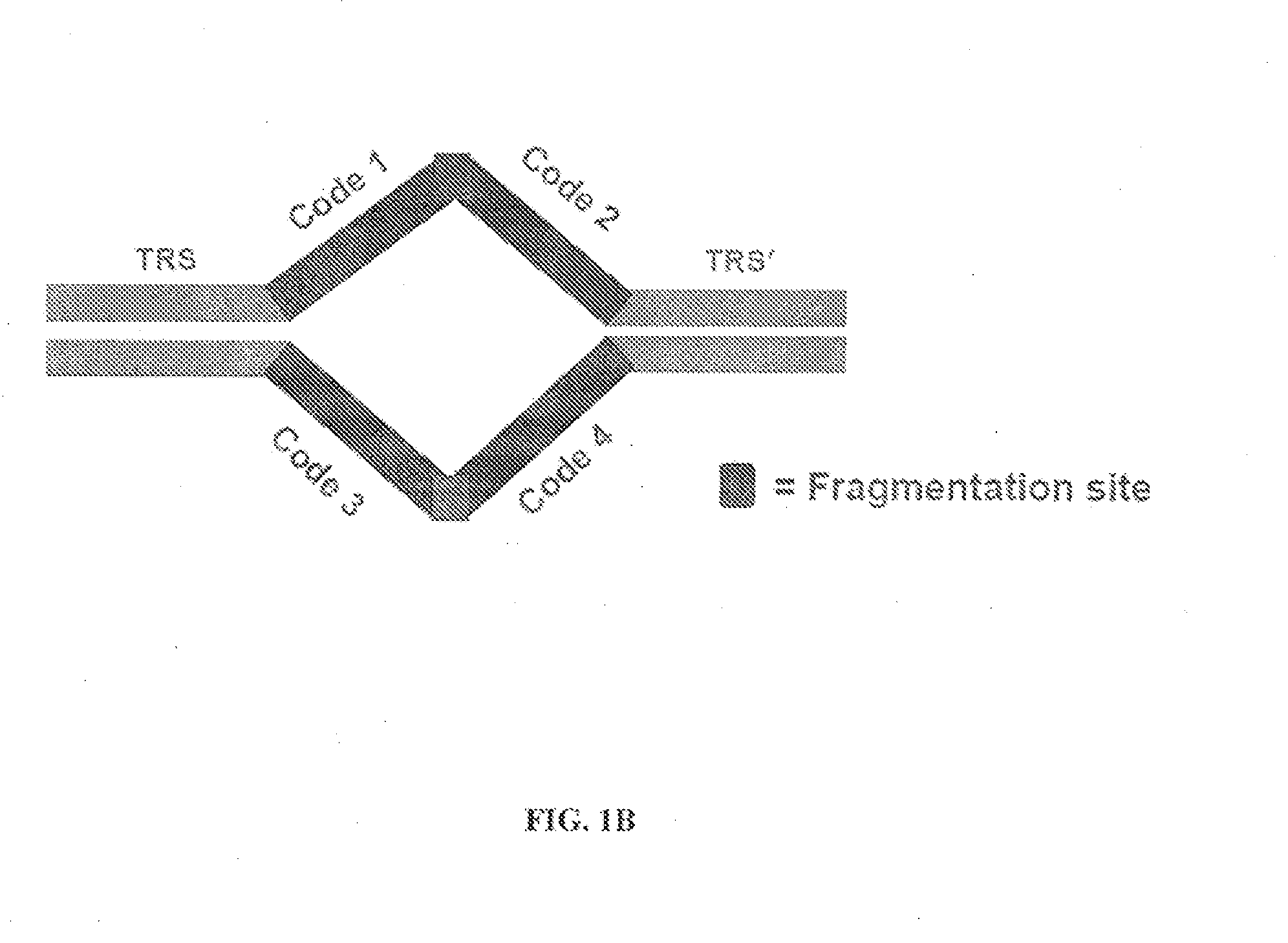
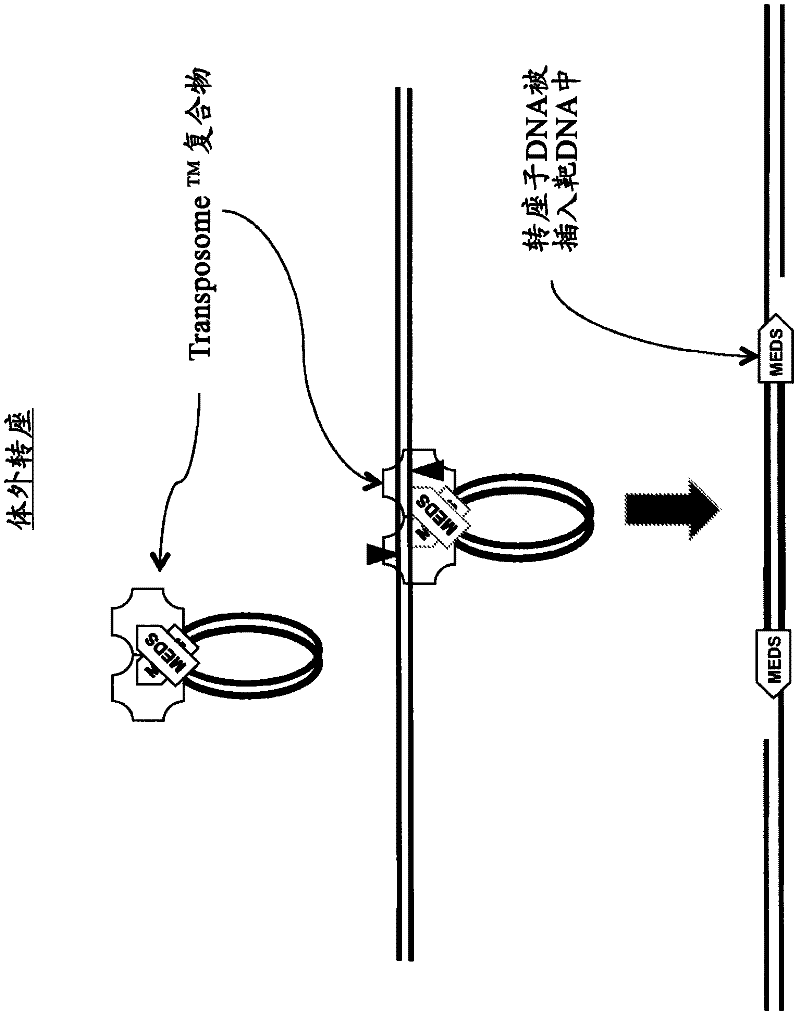
In certain embodiments, the present invention provides a way of “digitally” marking different the alleles of different chromosomes by using a transposase to insert differently barcoded transposons into genomic DNA before further analysis. According to this method, each allele becomes marked with a unique pattern of transposon barcodes. Because each unique pattern of transposon barcodes identifies a particular allele, the method facilitates determinations of ploidy and copy number variation, improves the ability to discriminate among homozygotes, heterozygotes, and patterns arising from sequencing errors, and allows loci separated by uninformative stretches of DNA to be identified as linked loci, thereby facilitating haplotype determinations. Also provided is a novel artificial transposon end that includes a barcode sequence in two or more positions that are not essential for transposition.
Owner:DIGENOMIX CORP
Gene regulation in transgenic animals using a transposon-based vector
InactiveUS7608451B2Improve efficiencyHigh copy numberEgg immunoglobulinsFood genetic modificationAnimal useTransgene
Administration of modified transposon-based vectors has been used to achieve stable incorporation of exogenous genes into animals. These transgenic animals produce transgenic progeny. Further, these transgenic animals produce large quantities of desired molecules encoded by the transgene. Transgenic egg-laying animals produce large quantities of desired molecules encoded by the transgene and deposit these molecules in the egg.
Owner:PROTEOVEC HLDG L L C
Enhanced sleeping beauty transposon system and methods for using the same
Methods and compositions for introducing a nucleic acid into the genome of a cell are provided. In the subject methods, a Sleeping Beauty transposon that includes the nucleic acid is introduced into the cell along with a source of a mutant Sleeping Beauty transposase that provides for enhanced integration as compared to the wild-type Sleeping Beauty transposase having an amino acid sequence as shown in SEQ ID NO:01. Introduction of the mutant Sleeping Beauty Transposase and transposon results in integration of the nucleic acid into the cell genome. Also provided are mutant transposases and transposons, as well as systems and kits thereof, that find use in practicing the subject methods. The subject methods and compositions find use in a variety of different applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Minimal piggybac vectors for genome integration
ActiveUS20160160235A1Reduce in quantityEfficient transpositionTransferasesFermentationGenomeDelivery system
Disclosed are genetic delivery systems that utilize genetic elements of the piggyBac family transposon system, and methods of introducing nucleic acid into target cells using the genetic delivery systems.
Owner:UNIV OF SOUTH ALABAMA
Linking sequence reads using paired code tags
InactiveUS20150259736A1Reduce in quantityMicrobiological testing/measurementLibrary member identificationComputational biologyTransposon element
Owner:ILLUMINA INC
Transposon end compositions and methods for modifying nucleic acids
The present invention provides the use of transposases and transposon ends to generate extensive fragmentation and 5′-tagging of double-stranded target DNA in vitro, followed by the use of DNA polymerases to generate 5′- and 3′-tagged single DNA without PCR amplification reactions. Methods, compositions and kits for stranded DNA fragments, wherein the first label on the 5' end shows the sequence of the transferred transposon end and optionally an additional arbitrary sequence, and the second label on the 3' end shows the same The first tab shows a sequence different from the sequence. The method can be used to generate 5' and 3' tagged DNA fragments for use in a variety of processes including metagenomic analysis of DNA in environmental samples, copy number variation (CNV) analysis of DNA, and including massively parallel DNA sequencing (so-called "next generation sequencing") involves the process of comparative genome sequencing (CGS).
Owner:EPICENT TECH CORP
Heirarchical assembly methods for genome engineering
InactiveUS20070004041A1Improve securityImprove propertiesStable introduction of DNAFermentationBiological bodyGenomic Stability
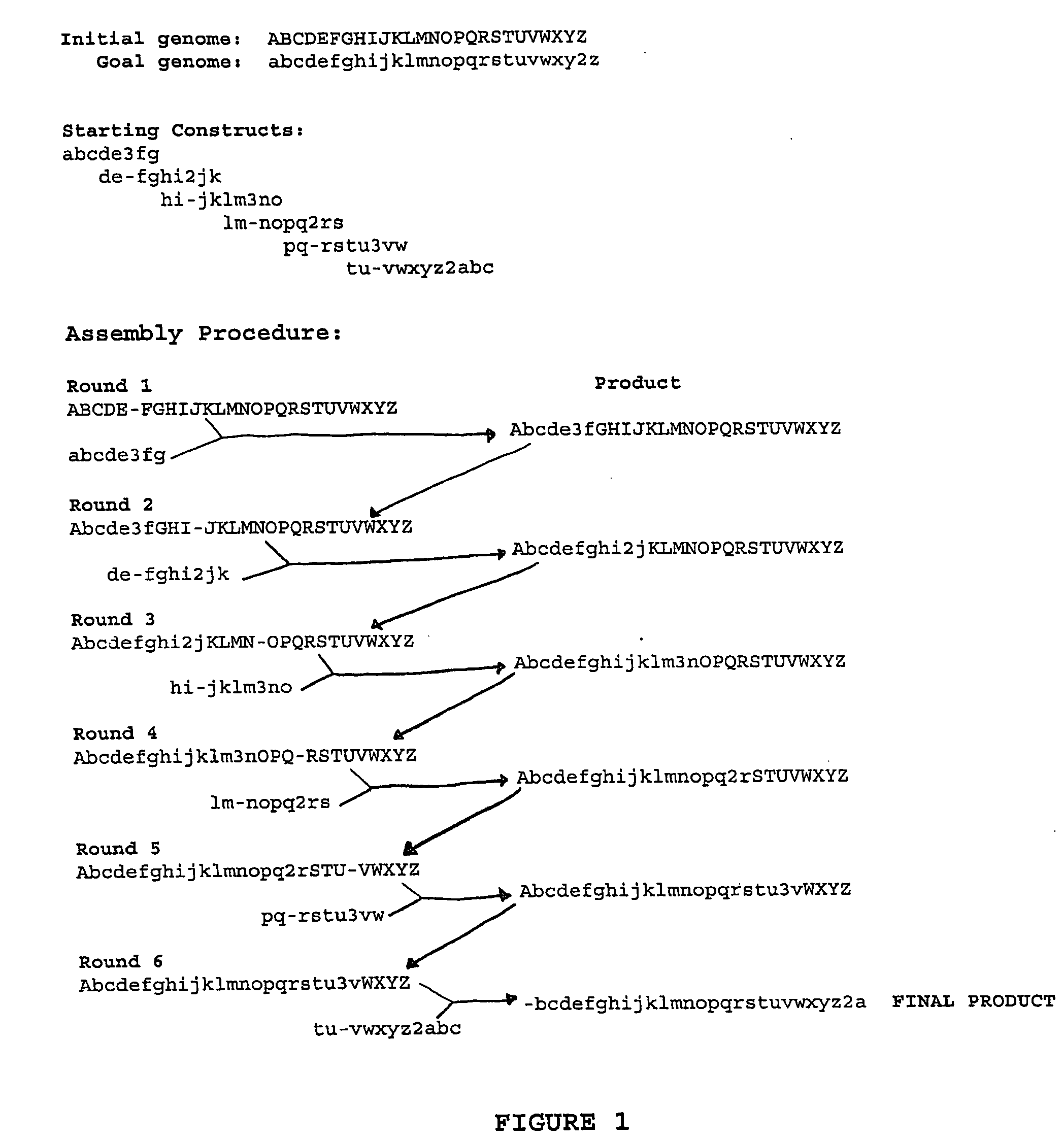
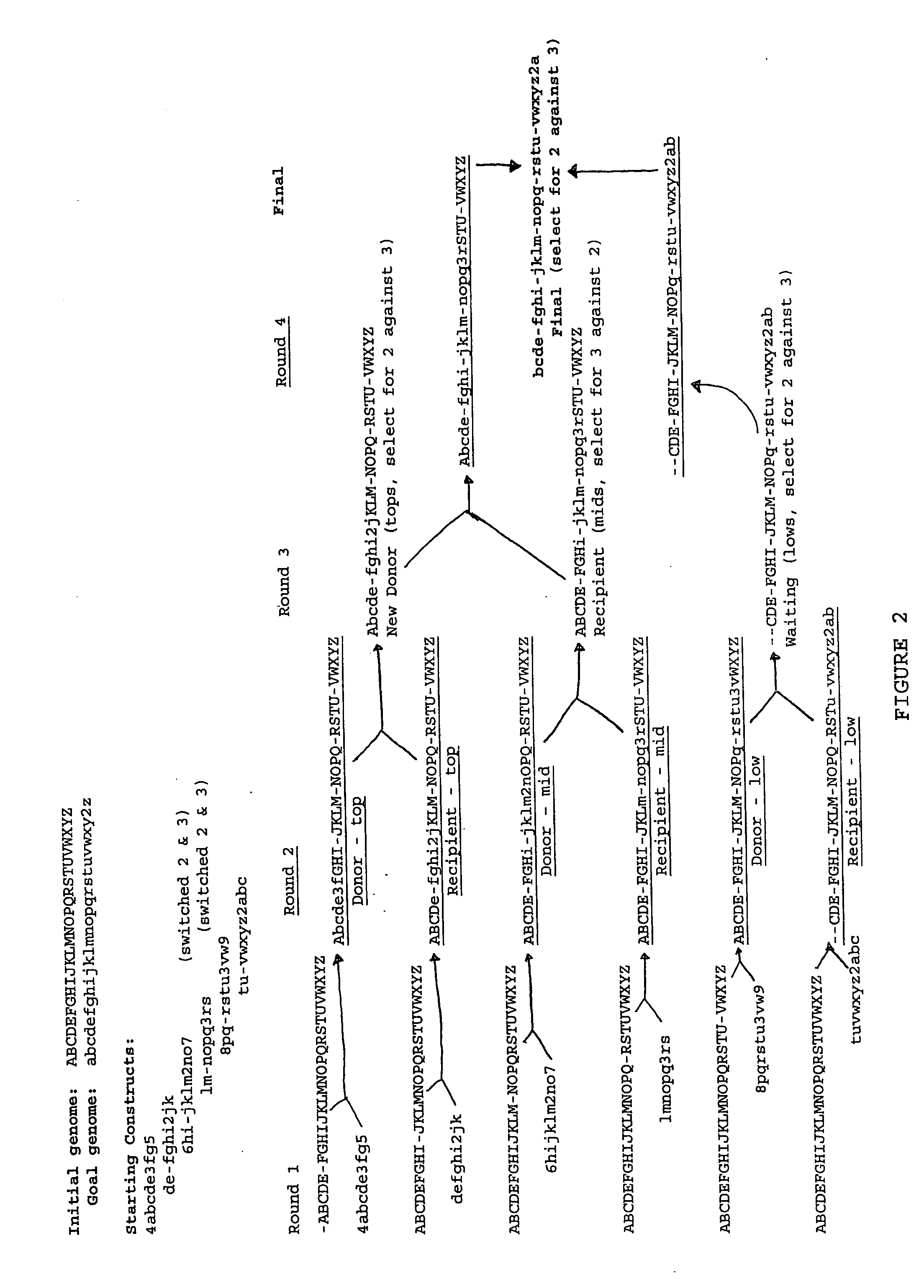
The present invention provides recombination based methods for assembling nucleic acids. In certain aspects the present invention provides hierarchical assembly methods for producing genome sized polynucleotide constructs. The methods may be used for assembling large polynucleotide constructs, for synthesizing synthetic genomes, or for introducing a plurality of nucleotide changes throughout the genome of an organism. In another aspect, the invention provides cells having increased genomic stability. For example, cells comprising alterations in at least a substantial portion of the transposons in the genome are provided.
Owner:CODON DEVICES
Methods and compositions for transposition using minimal segments of the eukaryotic transformation vector Piggybac
InactiveUS7105343B1Inserting DNA molecules into cells is enhancedEasy to insertSugar derivativesStable introduction of DNATransformation cellGene transfer
The present invention provides efficient transfer of genes into host cells or embryos to transform the cells or embryos by transposition vectors using the minimal amount of nucleotide sequences in the transposon piggyBac required for gene transfer. The transformed cells or embryos may also be developed into transgenic organisms.
Owner:UNIV OF NOTRE DAME DU LAC
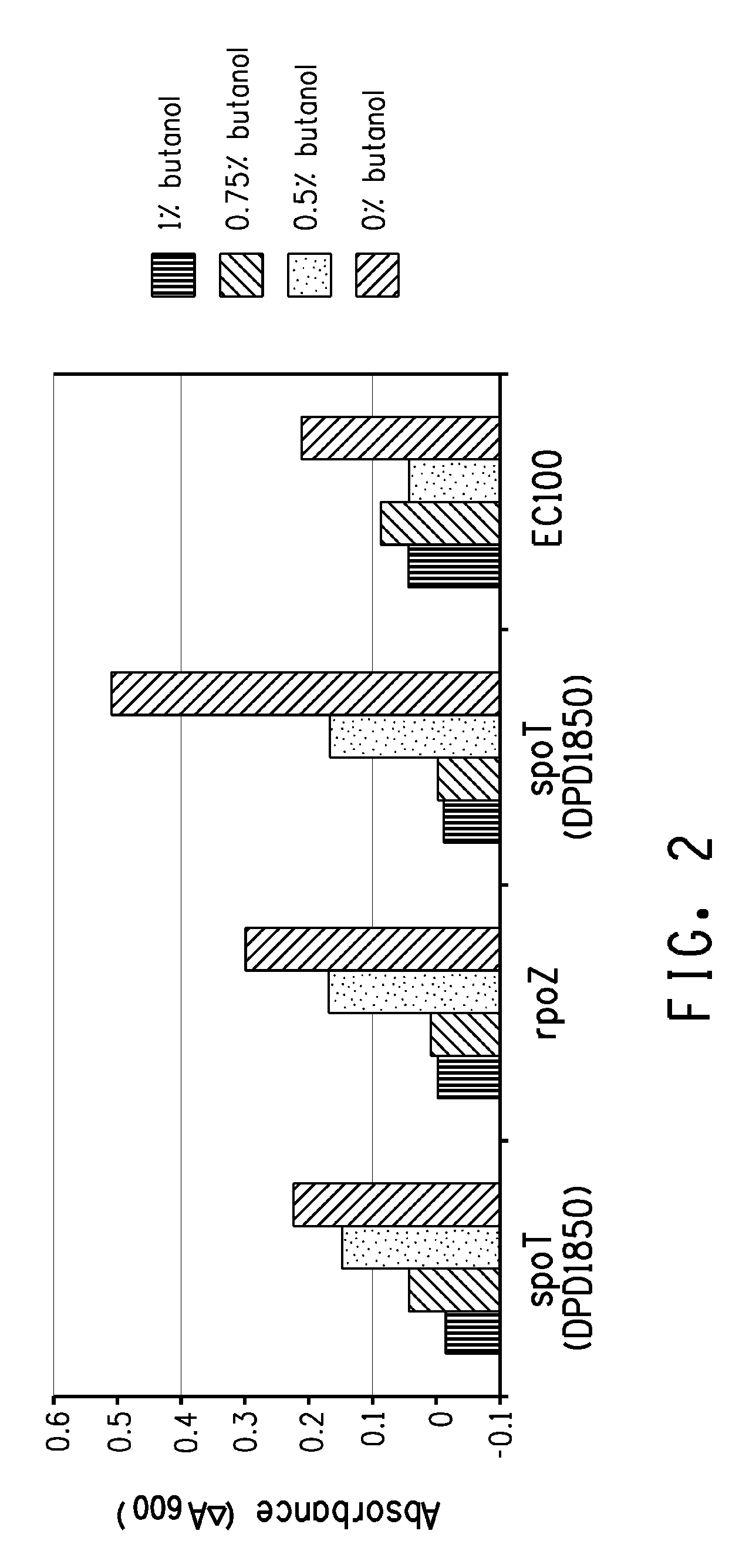
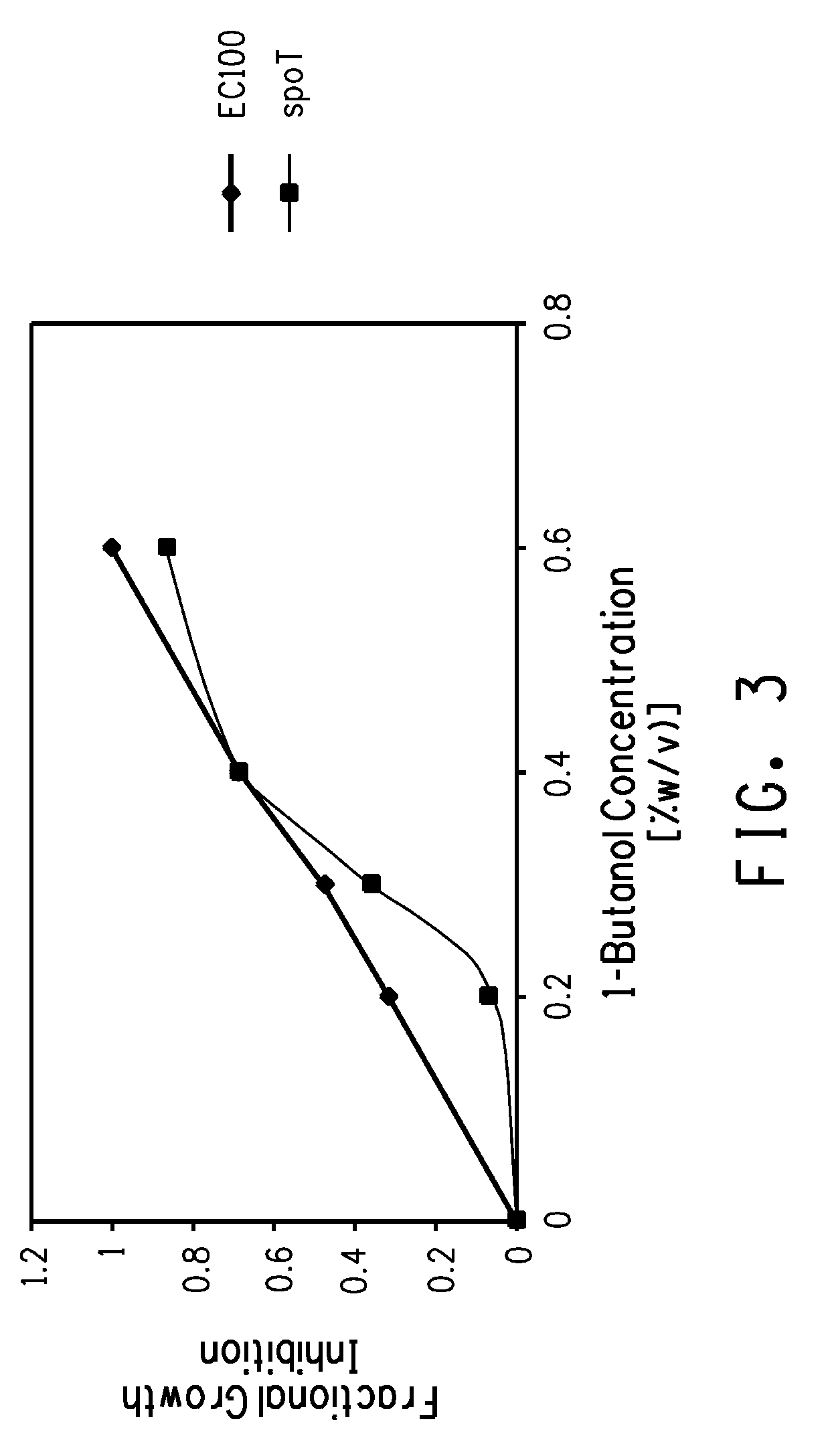
Production of four carbon alcohols using improved strain
Owner:GEVO INC
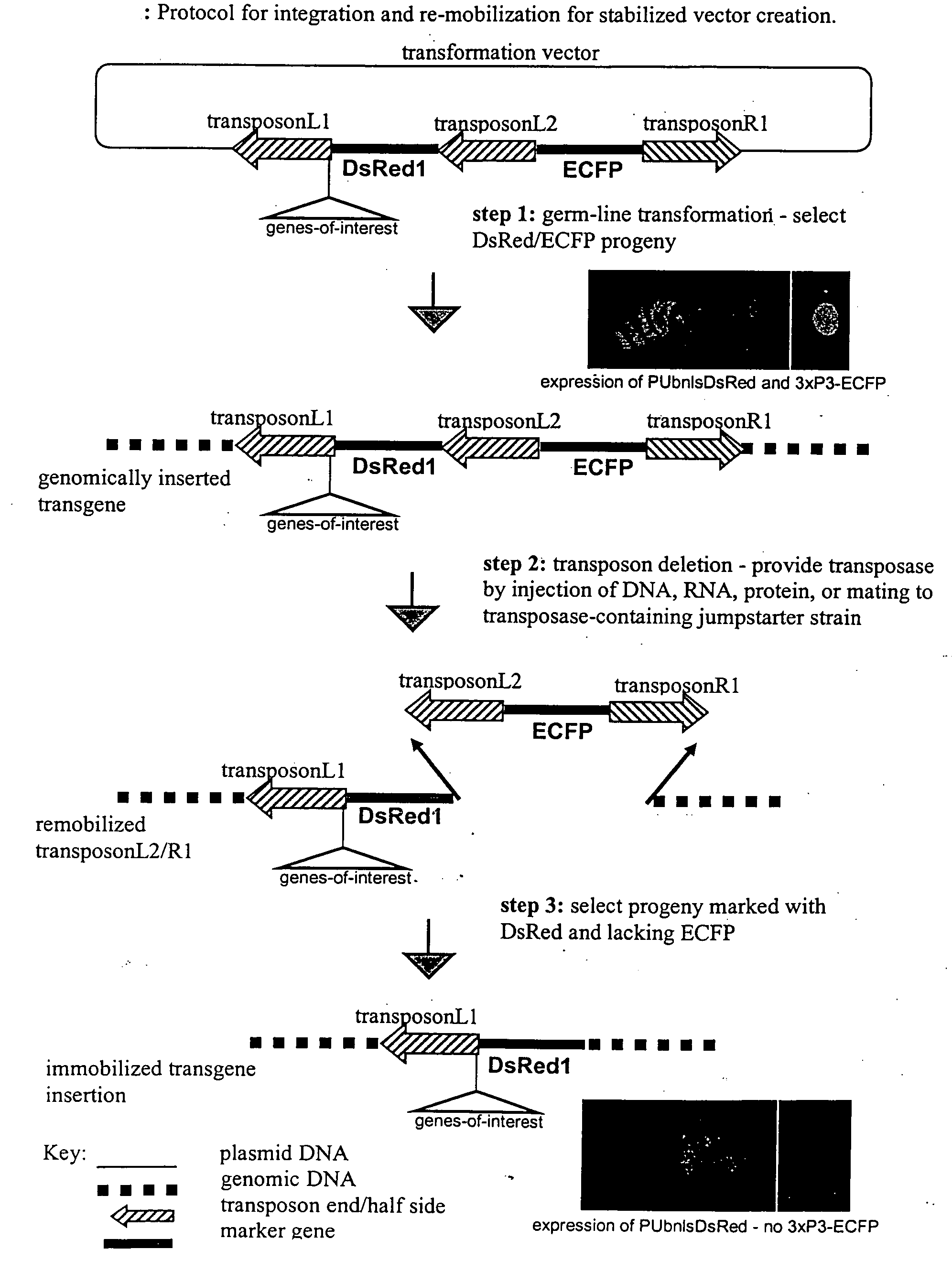
Systems for gene targeting and producing stable genomic transgene insertions
InactiveUS20090083870A1Improve stabilityStable introduction of DNANucleic acid vectorInstabilityTransgene
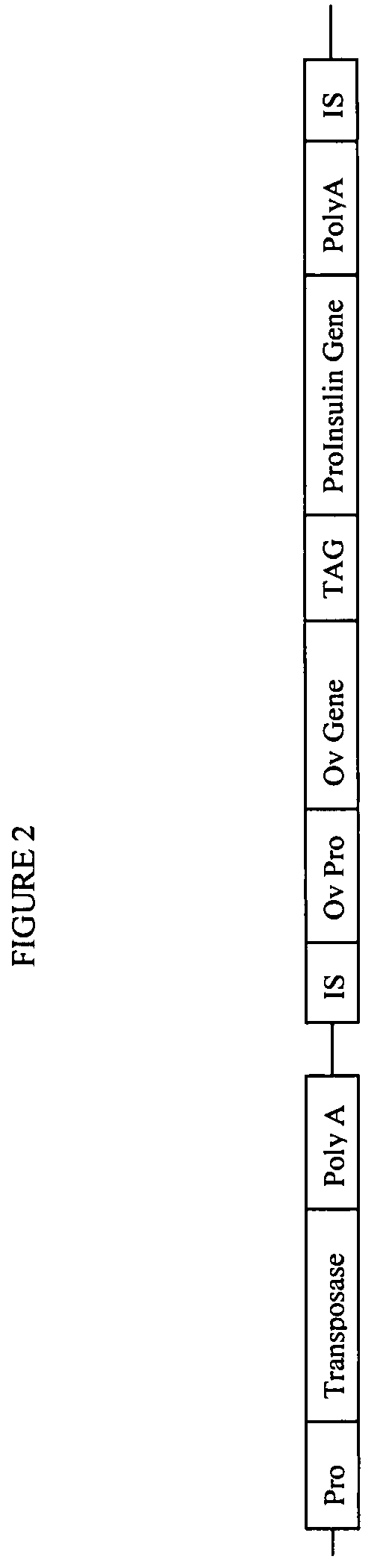
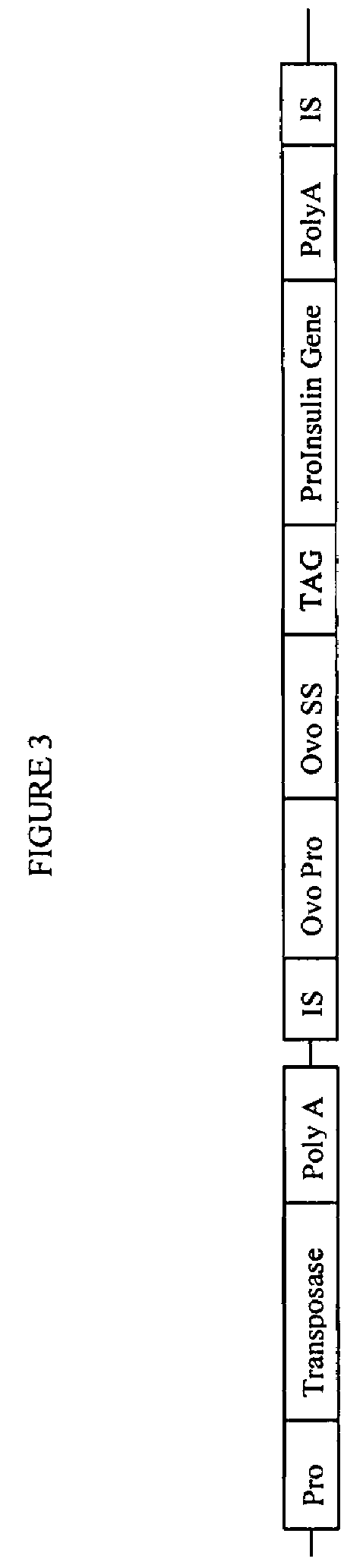
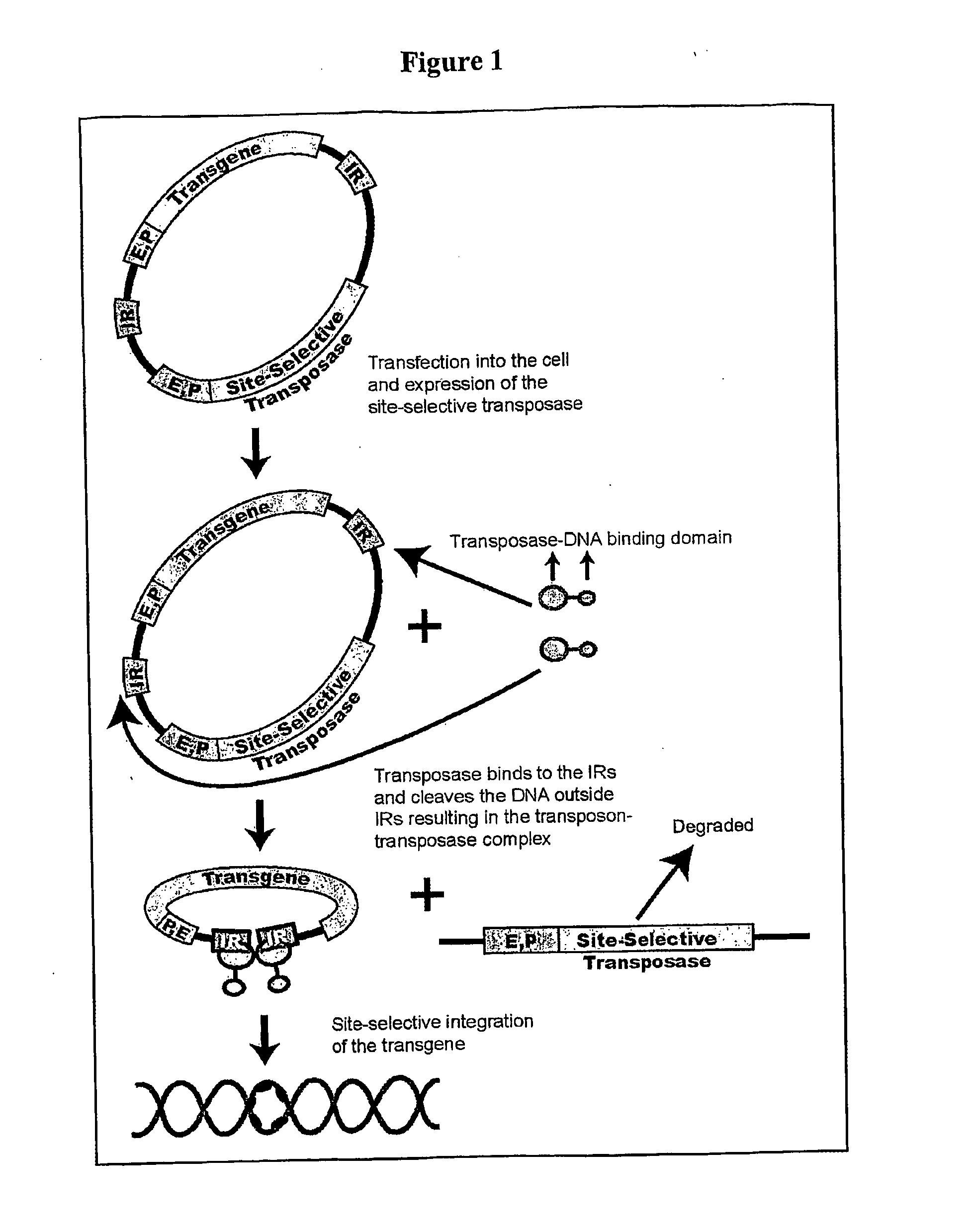
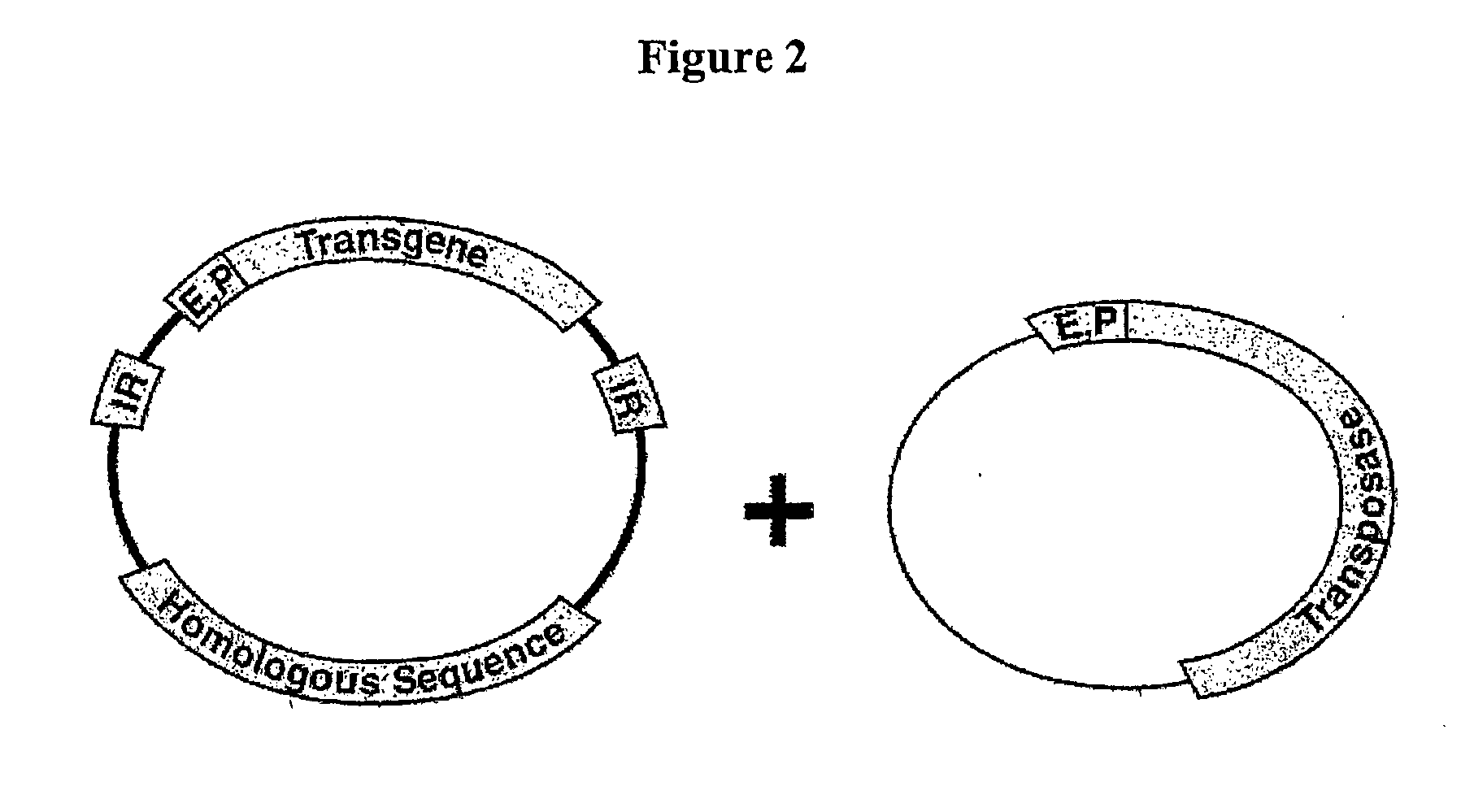
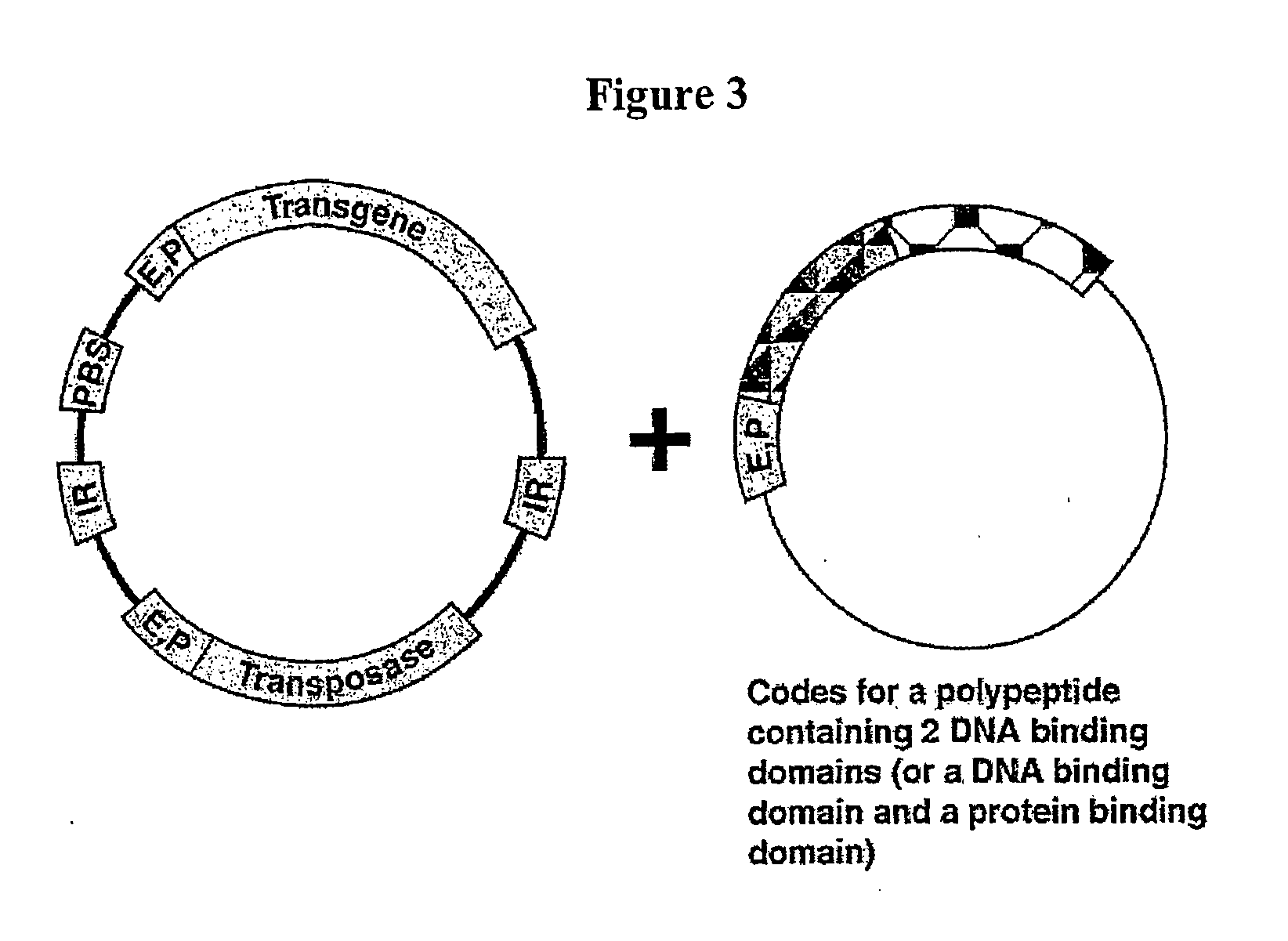
The novel germ-line transformation systems disclosed in this patent application allow the physical deletion of transposon DNA following the transformation process, and the targeting of transgene integrations into predefined target sites. In this way, transposase-mediated mobilization of genes-of-interest is excluded mechanistically and random genomic integrations eliminated. In contrast to conventional germ-line transformation technology, our systems provide enhanced stability to the transgene insertion. Furthermore, DNA sequences required for the transgene modification (e.g. transformation marker genes, transposase or recombinase target sites), are largely removed from the genome after the final transgene insertion, thereby eliminating the possibility for instability generated by these processes. The RMCE technology, which is disclosed in this patent application for invertebrate organisms (exemplified in Drosophila melanogaster) represents an extremely versatile tool with application potential far beyond the goal of transgene immobilization. RMCE makes possible the targeted integration of DNA cassettes into a specific genomic loci that are pre-defined by the integration of the RMCE acceptor plasmid. The loci can be characterized prior to a targeting experiment allowing optimal integration sites to be pre-selected for specific applications, and allowing selection of host strains with optimal fitness. In addition, multiple cassette exchange reactions can be performed in a repetitive way where an acceptor cassette can be repetitively exchanged by multiple donor cassettes. In this way several different transgenes can be placed precisely at the same genomic locus, allowing, for the first time, the ability to eliminate genomic positional effects and to comparatively study the biological effects of different transgenes.
Owner:HORN CARSTEN +1
Method of preparing transgenic organism with use of methylation and system therefor
InactiveUS20070022485A1Improve conversion efficiencyEfficient productionAnimal cellsHydrolasesGene conversionOrganism
A technique for efficiently introducing a foreign gene into cells with the use of transposons. In particular, a technique for efficiently preparing a transgenic organism with the use of a transposon having its transposition activity strikingly enhanced through methylation of a sequence containing the transposon. The methylation is retained even after incorporation in a genome, and now can be utilized in actual gene incorporation in a genome. This technique can realize strikingly efficient gene transformation as compared with the a method of preparing a transgenic organism with the use of conventional transposons.
Owner:JAPAN SCI & TECH CORP
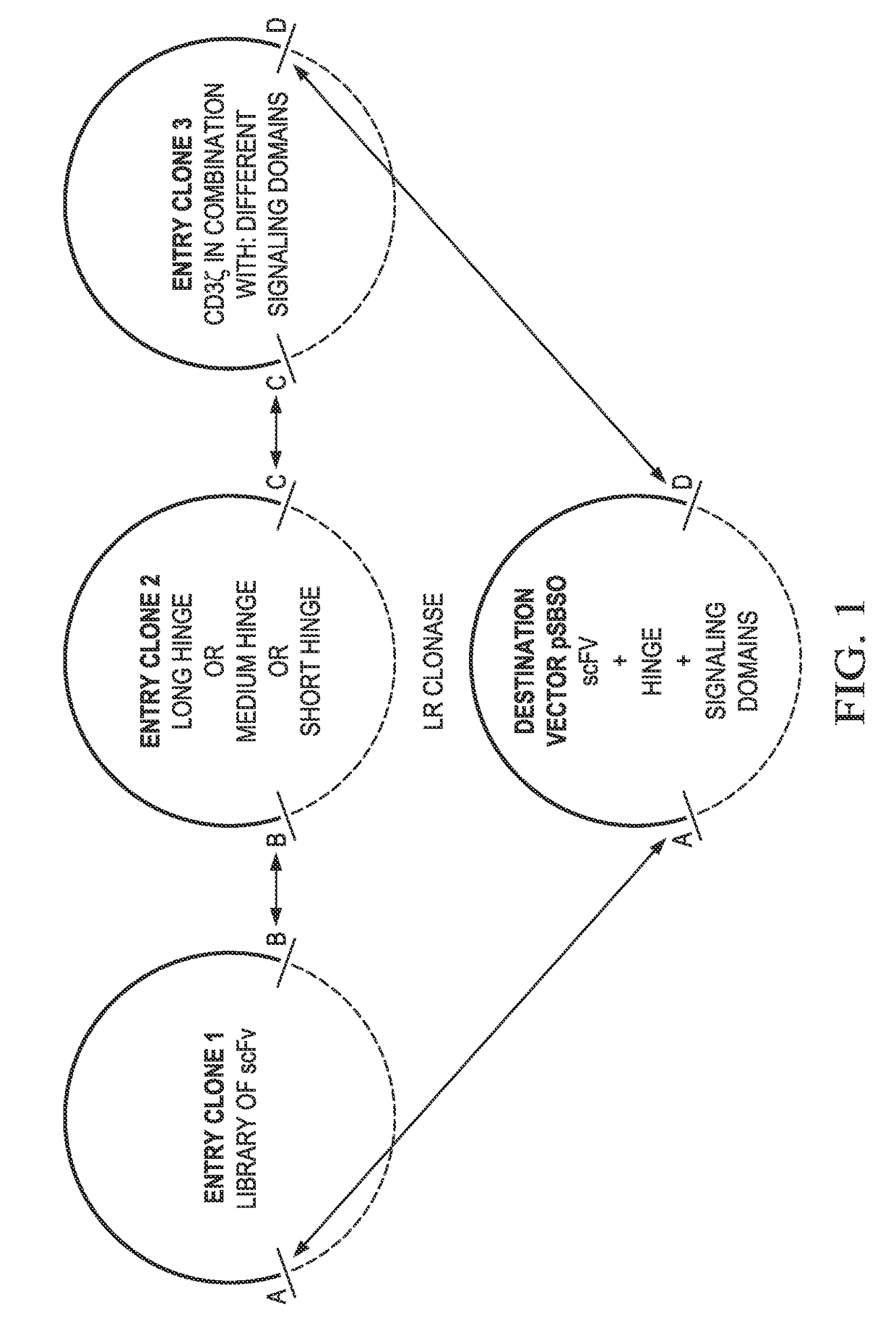
Chimeric antigen receptors and methods of making
ActiveUS20170183407A1Improved therapeutic potentialConvenient treatmentAntibacterial agentsTumor rejection antigen precursorsAntigen receptorsBinding domain
Provided are methods of generating chimeric antigen receptors (CAR). In some embodiments, library screening of CAR is performed by generating a vector encoding the CAR from random attachment of vectors from libraries of vectors encoding antigen-binding domains (e.g., scFv regions), hinge regions, and endodomains. In some embodiments, the vectors contain a transposon.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
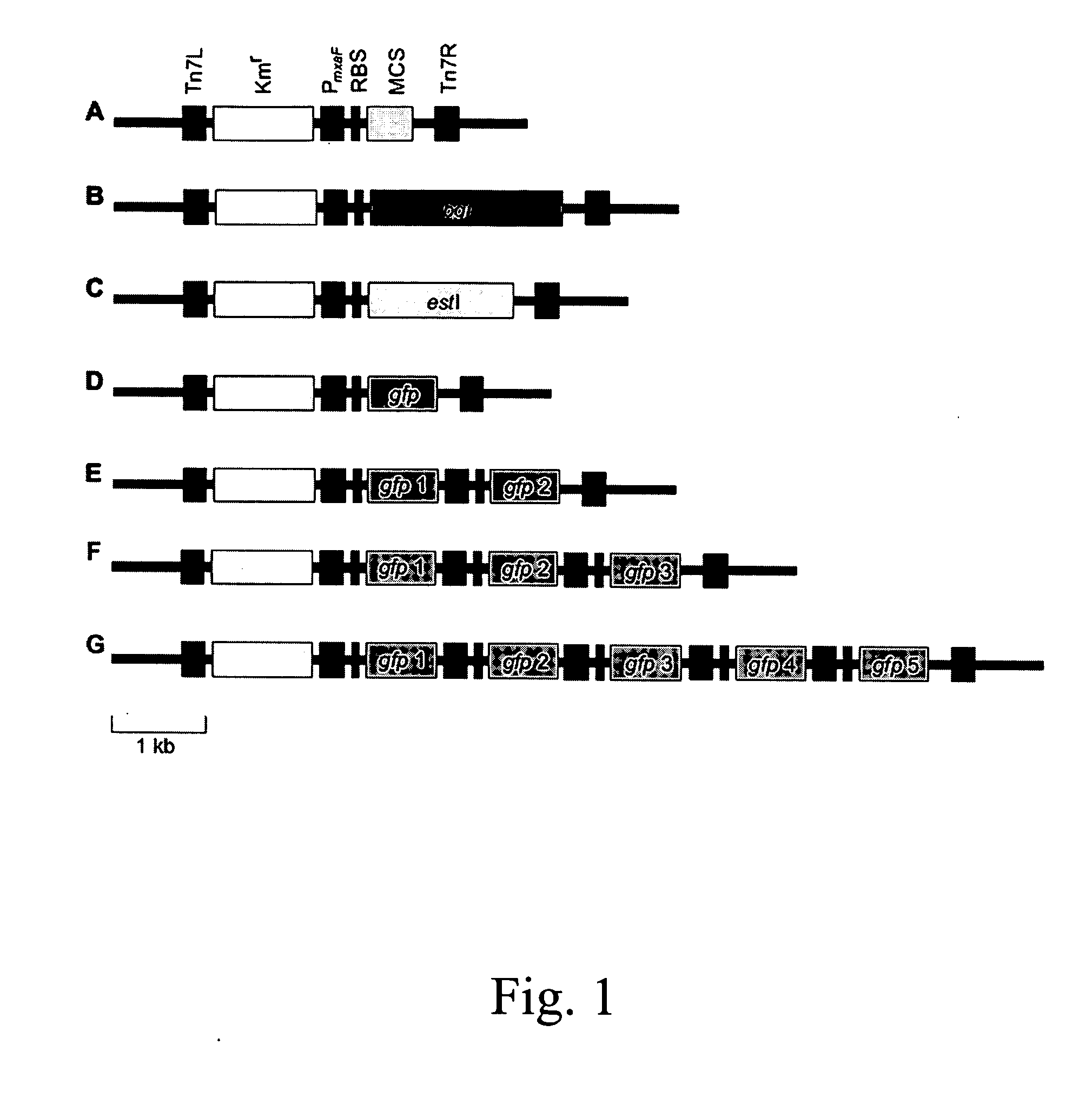
Multicopy-integration of heterologous genes and expression in methylobacterium
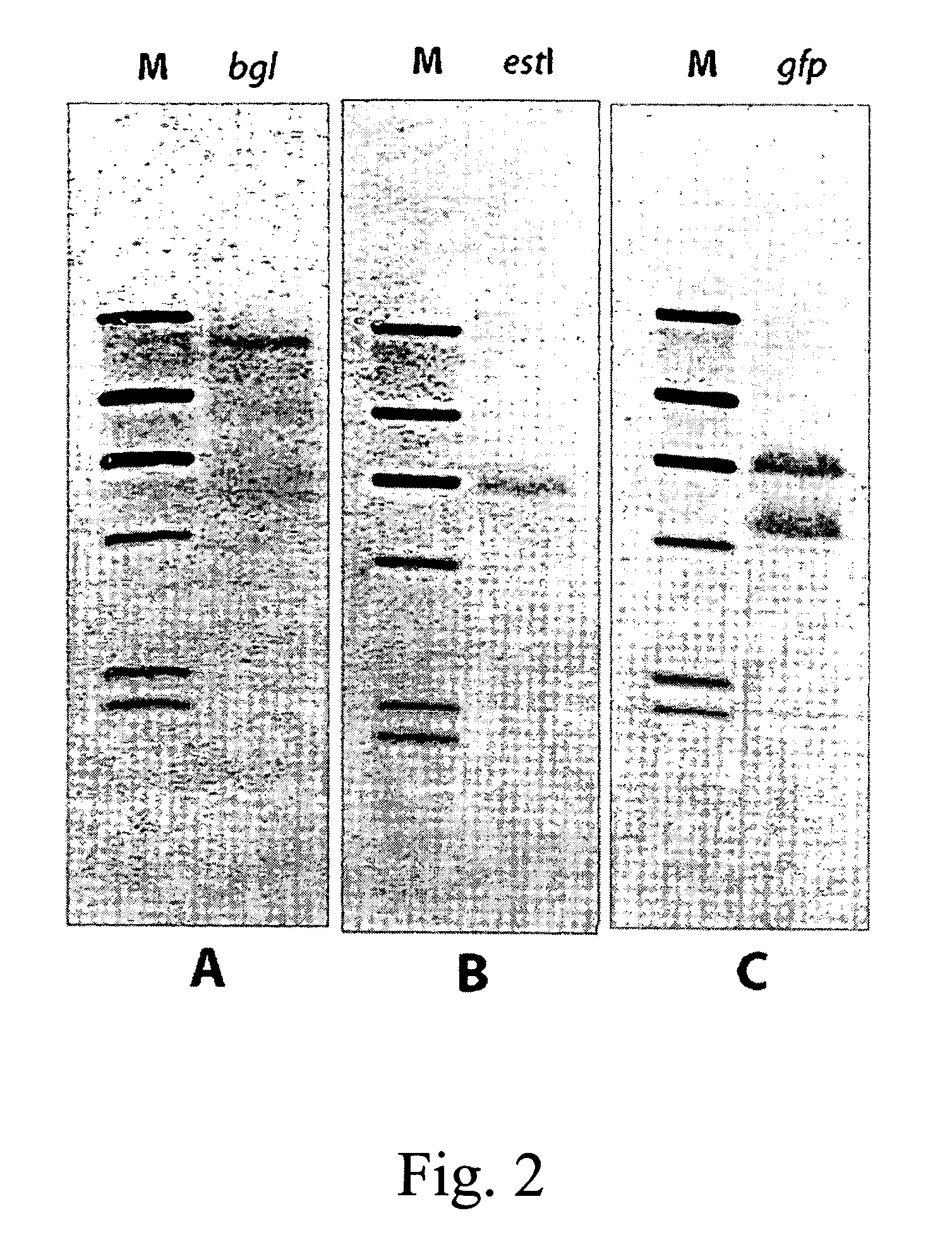
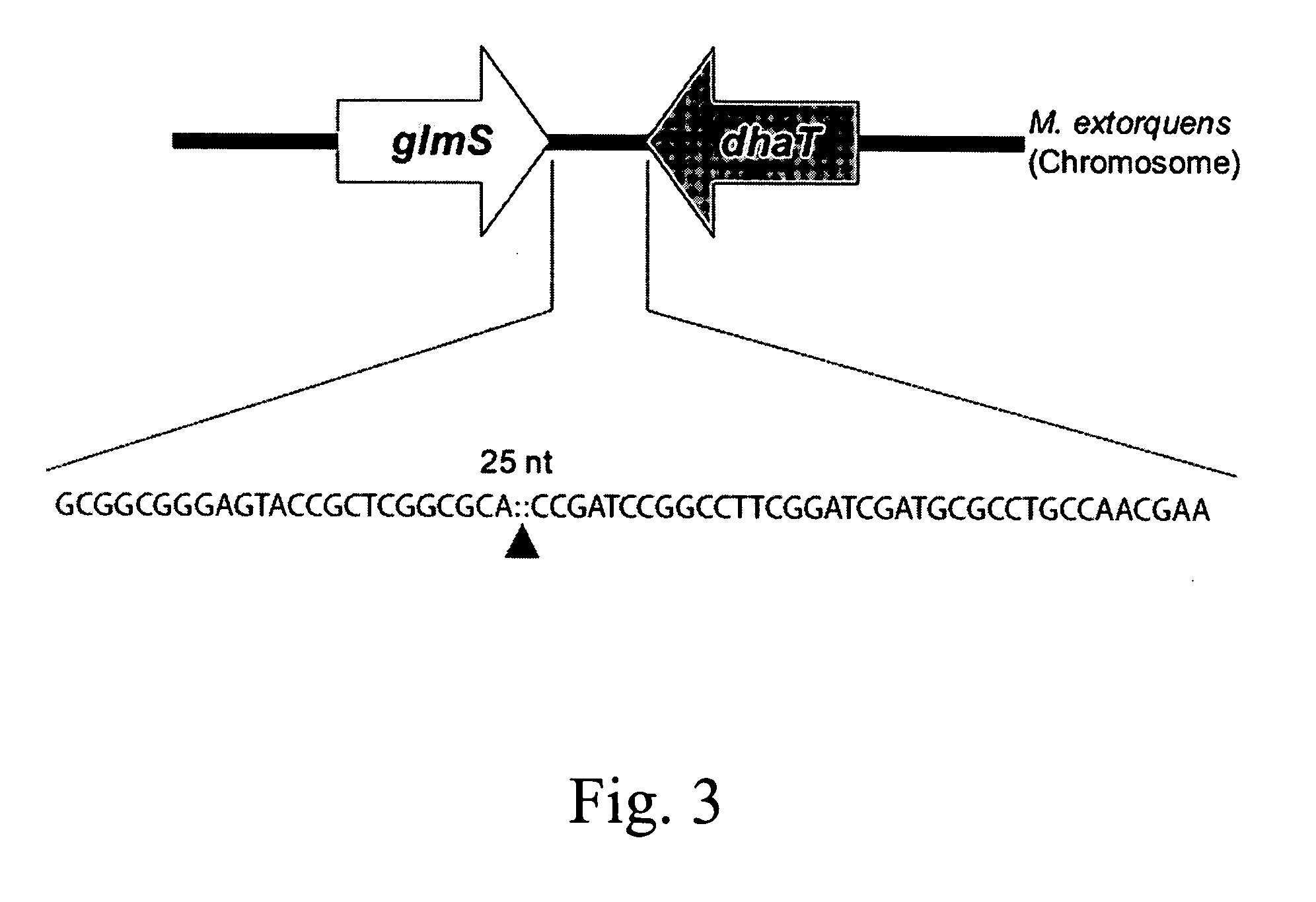
The integration of genes into methylobacterium, such as M. extorquens ATCC 55366 is disclosed, using a transposon system, preferably the mini-Tn7 transposon system, and under the control of a promoter, such as the strong methanol dehydrogenase promoter (PmxaF). Multicopy integration of genes of interest is also disclosed. The unique and specific attachment site for the Tn7 attachment (attTn7) is identified for M. extorquens.
Owner:NAT RES COUNCIL OF CANADA
Silk Thread Containing Spider Thread Protein and Silk Worm Producing the Silk Thread
InactiveUS20080287651A1Maintain good propertiesHigh strengthPeptide/protein ingredientsImmunoglobulinsBiotechnologyHigh intensity
A transgenic silkworm having transferred therein a gene which encodes spider thread protein having desired properties of high strength and high elasticity while leaving the silkworm fibroin H chain gene intact, by means of utilizing a transposon function, is used to produce in the transgenic silkworm a spider thread protein having the desired properties without lowering the strength or elasticity of silk thread produced by the transgenic silkworm, thereby providing hybrid silk of spider and silk threads having the desired properties.
Owner:TORAY IND INC +1
Human application of engineered chimeric antigen receptor (CAR) t-cells
ActiveUS20160158285A1Easy SurvivalInhibit expressionMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseElectroporation
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising a chimeric antigen receptor (CAR). In particular aspects, CAR-expressing T-cells are producing using electroporation in conjunction with a transposon-based integration system to produce a population of CAR-expressing cells that require minimal ex vivo expansion or that can be directly administered to patients for disease (e.g., cancer) treatment.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Transposon-based vectors and methods of nucleic acid integration
Disclosed herein are compositions comprising integrating enzymes that can deliver nucleic acids to a target DNA. Additionally, the methods of using the compositions disclosed herein relate to treatments for a variety of infections, conditions, and genetic disorders.
Owner:VANDERBILT UNIV +1
Stable knockout single plasmid vector with coordination of transposon and CRISPR/Cas9 system and application of stable knockout single plasmid vector
InactiveCN107686848ASimplify the build processThe process is simpleHydrolasesStable introduction of DNAStable cell lineBiology
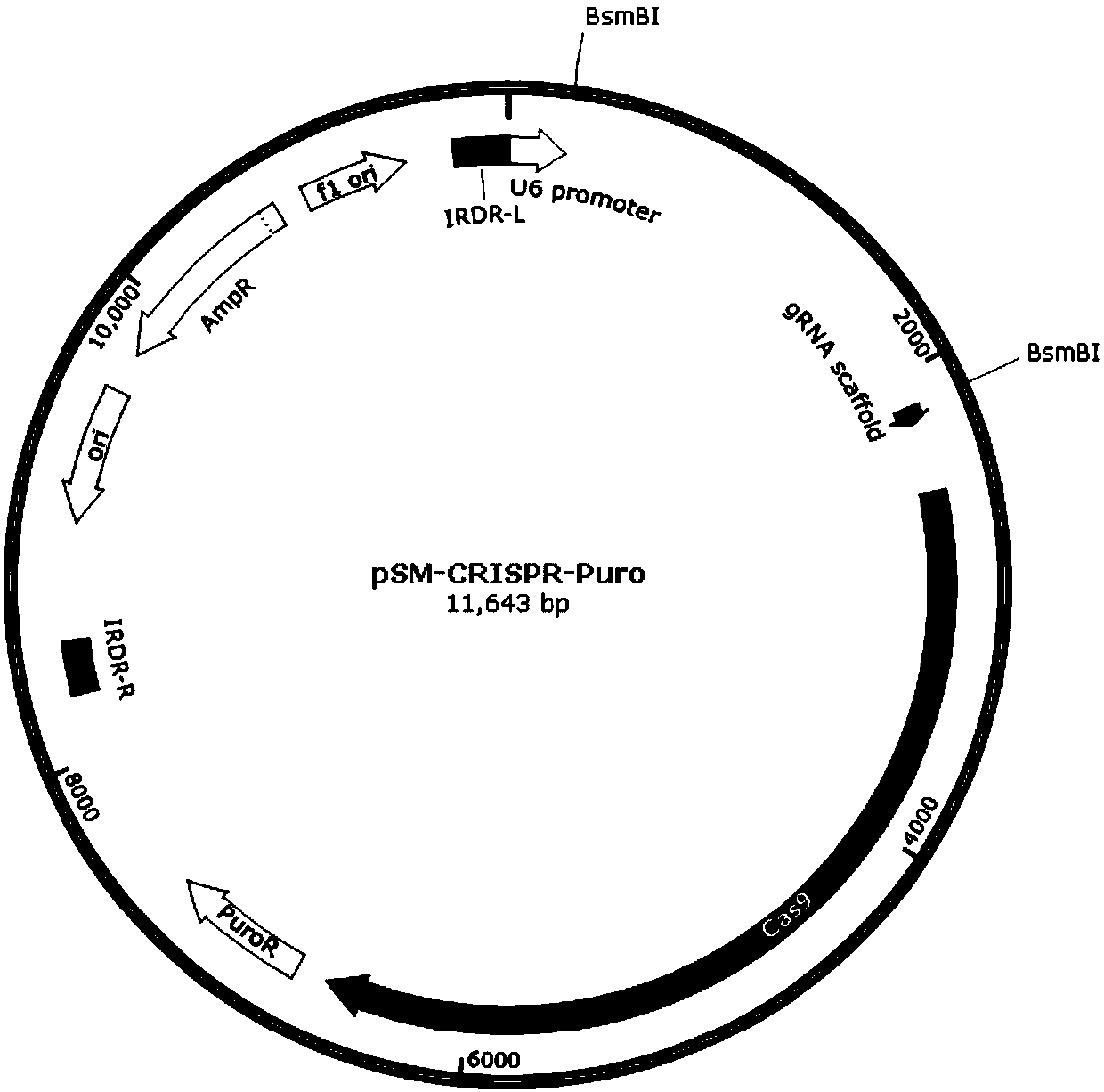
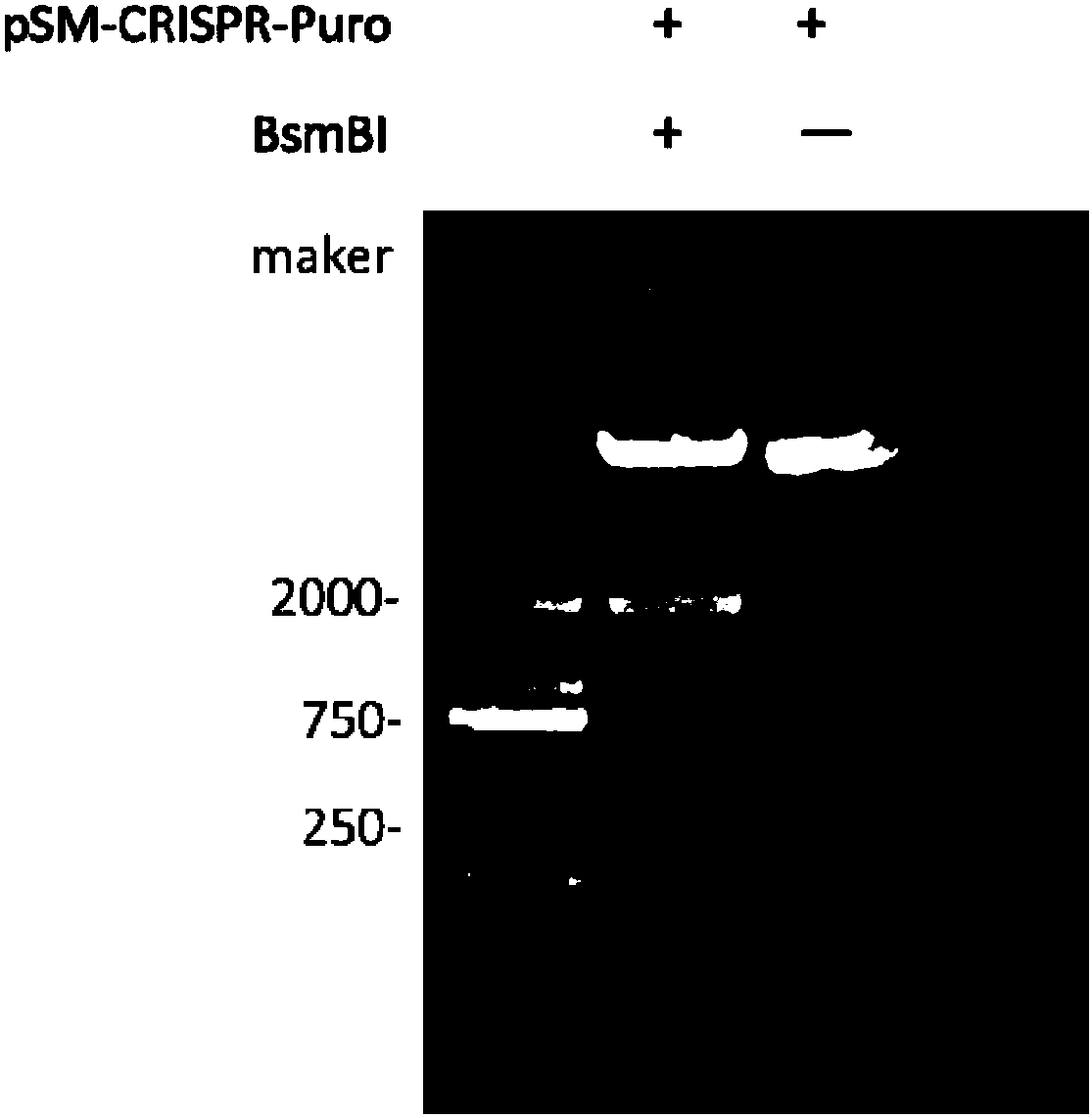
The invention discloses a stable knockout single plasmid vector with coordination of transposon and a CRISPR / Cas9 system and application of stable knockout single plasmid vector and belongs to the field of gene engineering. The single plasmid vector is a double-stranded circular plasmid containing an IRDR-L-IRDR-R box, and the IRDR-L-IRDR-R box comprises an IRDR-L sequence, a promoter, a gRNA scaffold sequence, a Cas9 protein sequence, a resistance screening gene sequence and an IRDR-R sequence. The single plasmid vector provided by the invention can realize expression of sgRNA and a Cas9 protein only needing once construction and once transfection. A method is simple in process, is efficient and rapid, greatly simplifies a plasmid construction flow, shortens an experiment period and improves the working efficiency. The vector provided by the invention carries a transposase recognition sequence, does not use a virus, can conveniently, rapidly and safely establish a gene knockout stablecell line and is beneficial to screening stable cell lines by comprising the puromycin screening resistance.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com