Method for synthesizing quinolone medicaments
A synthetic method and quinolone technology, applied in the direction of organic chemistry, etc., can solve the problems of increasing post-processing procedures and related processing equipment, increasing the difficulty of safety production management, increasing material types and unit consumption, etc., to solve the problem of environmental protection, odor, The effect of shortening the recovery time and simplifying the production operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
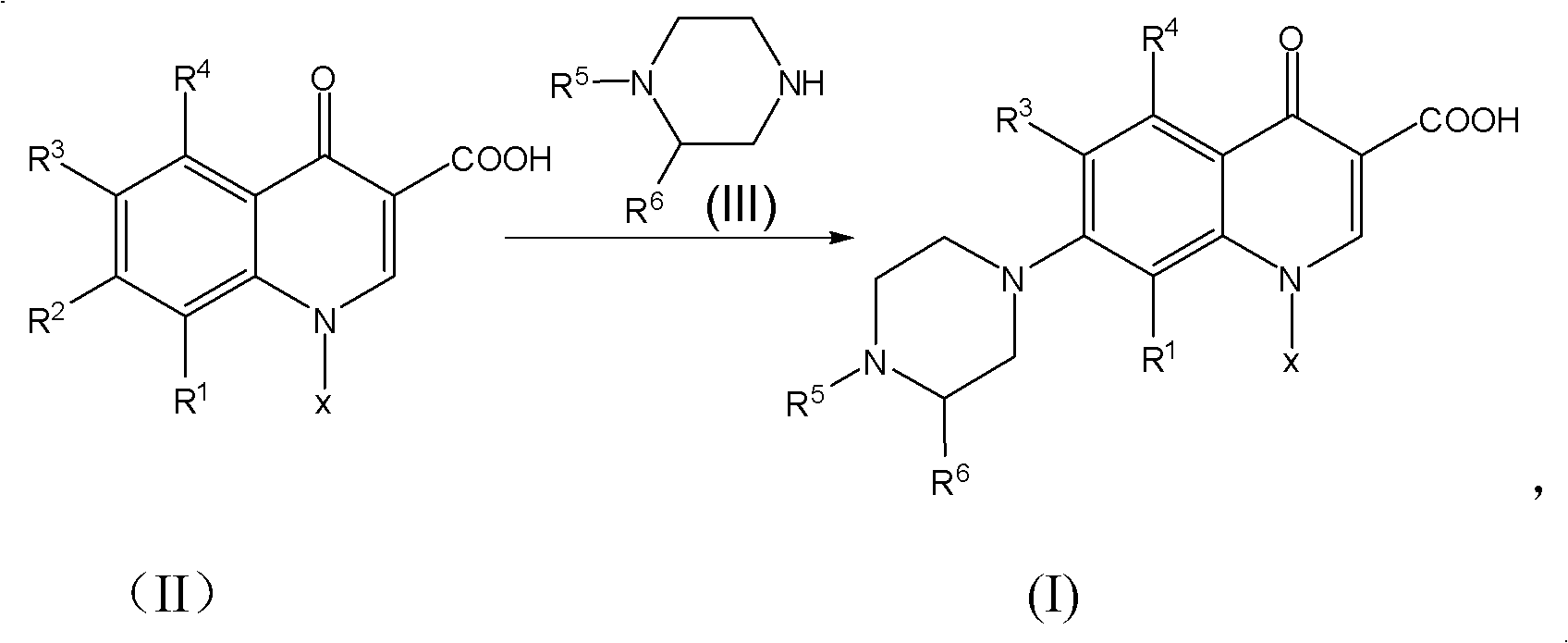
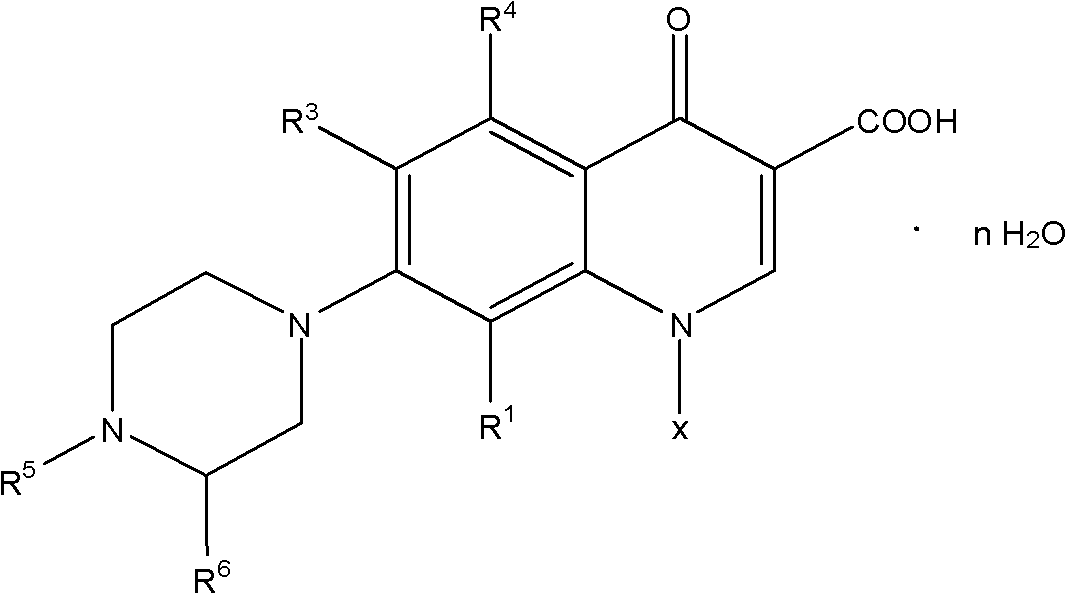
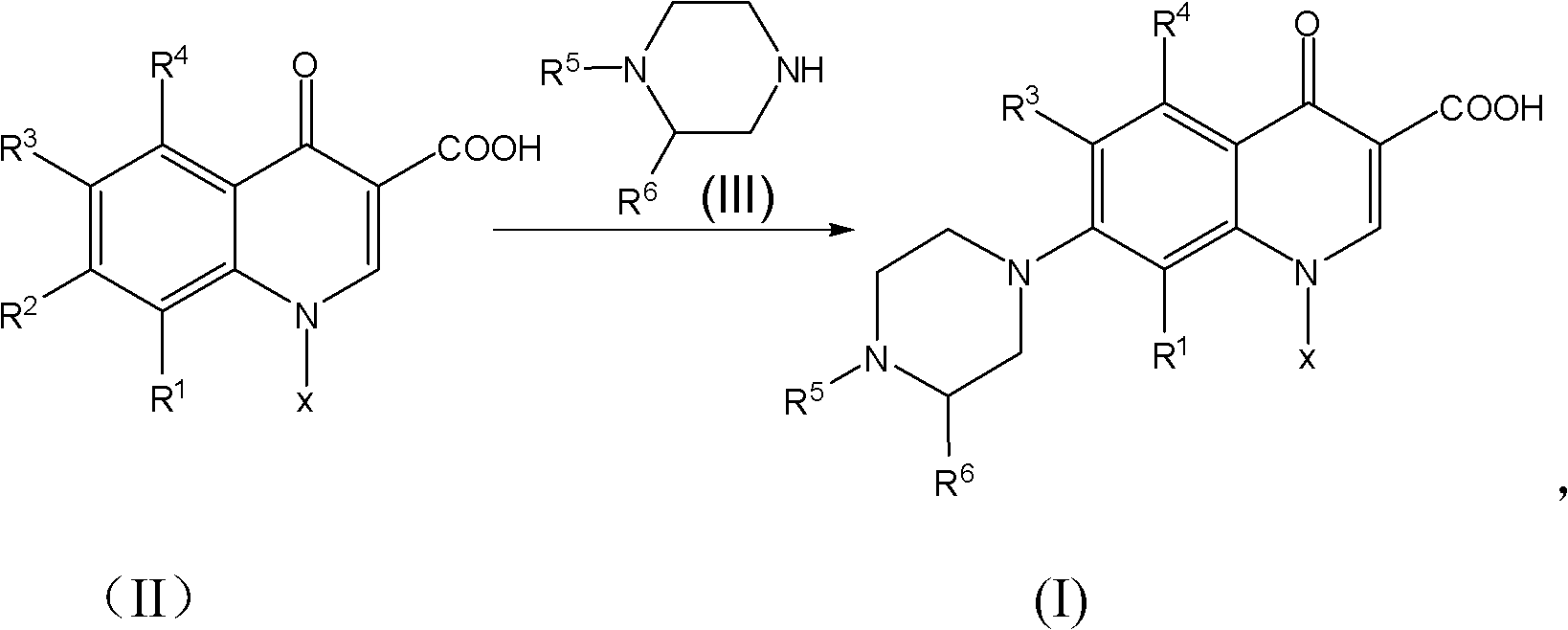
[0049] Weigh respectively 25g levofluorocarboxylic acid (0.0889 mole), 33g N-methylpiperazine (0.3294 mole), 0.2g cerium trichloride heptahydrate and 12g water (0.6666 mole), drop into 250ml reaction bottle successively, Stir, heat up to 97°C, and keep the reaction at 97±2°C for 9 hours, and monitor the completion of the reaction by HPLC. After shrinking the piperidine, recover the N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90~110°C and -0.09~-0.095MPa until there is basically no flow, then add 160ml of methanol to the reaction bottle, heat and reflux for 0.5 hours, and cool down to At about 5°C, keep warm at 5-8°C for 1 hour, filter with suction, rinse the filter cake with an appropriate amount of methanol until the filtrate is colorless, and drain it. Put the obtained pale yellow solid in a 500ml flask, add 160g of purified water, stir and beat for 45 minutes, then add 30% liquid caustic soda dropwise into the bottle, stir at 25±1°C un...
Embodiment 2
[0051] Weigh respectively 25g levofluorocarboxylic acid (0.0889 mole), 30g N-methylpiperazine (0.2995 mole), 0.2g tetrabutylammonium bromide and 11g water (0.6111 mole), drop into 250ml reaction bottle successively, stir , heated to 100°C, and kept at 102±2°C for 8 hours, and monitored by HPLC to complete the reaction. After shrinking the piperidine, recover the N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90~110°C and -0.09~-0.095MPa until there is basically no flow, then add 160ml of acetone to the reaction bottle, heat and reflux for 0.5 hours, and cool down to At about 5°C, keep warm at 5-8°C for 1 hour, filter with suction, rinse the filter cake with an appropriate amount of acetone until the filtrate is colorless, and then drain it. Put the obtained pale yellow solid in a 500ml flask, add 160g of purified water, stir and beat for 45 minutes, then add 30% liquid caustic soda dropwise into the bottle, stir at 25±1°C until clear, and th...
Embodiment 3
[0053] Weigh respectively 25g levofluorocarboxylic acid (0.0889 mole), 37g N-methylpiperazine (0.3694 mole), 0.2g N, N-carbonyldiimidazole and 12g water (0.6666 mole), drop into 250ml reaction bottle successively, stir , heated to 105°C, and kept at 104±1°C for 10 hours, and monitored by HPLC to complete the reaction. After shrinking the piperidine, recover the N-methylpiperazine aqueous solution under reduced pressure under the conditions of 90-110°C and -0.09-0.095MPa until there is basically no flow, then add 180ml of ethyl acetate to the reaction bottle, and heat to reflux for 0.5 hours. Cool down to about 5°C, keep warm at 5-8°C for 1 hour, filter with suction, rinse the filter cake with an appropriate amount of ethyl acetate until the filtrate is colorless, and drain it. Put the obtained pale yellow solid in a 500ml flask, add 160g of purified water, stir and beat for 45 minutes, then add potassium hydroxide solution dropwise into the bottle, stir at 25±1°C until clear, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com