Preparation method of pimavanserin
A technology of pimavanserin and formamide, applied in the field of medicinal chemistry, can solve the problems of causing pulmonary edema, difficult reaction control, increasing the risk of operators, etc. Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
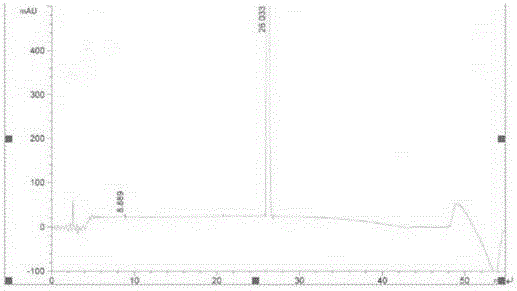
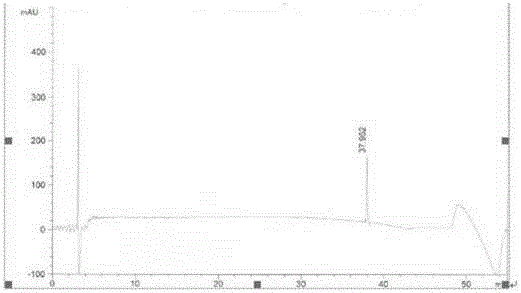
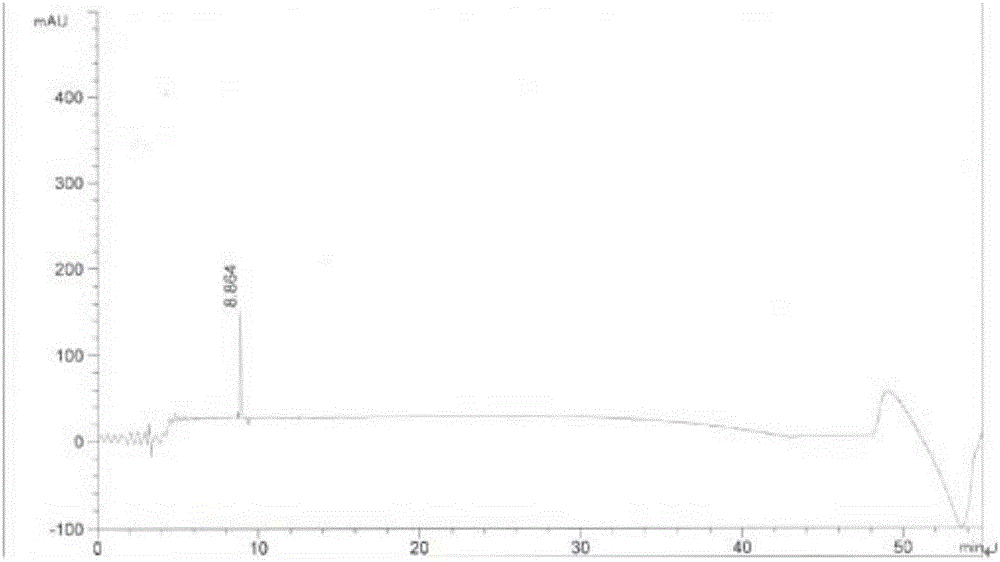
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1N-(4-isobutoxyphenyl)-1H-imidazole-1-carboxamide (intermediate 2)
[0037] Add 100g (0.558mol) of 4-isobutoxybenzylamine to 500ml of toluene, stir to dissolve, add 100g (0.627mol) of carbonyldiimidazole at room temperature, and stir at room temperature for 1 to 2 hours to obtain N-(4-isobutyl The toluene solution of oxyphenyl)-1H-imidazole-1-carboxamide was directly used in the next reaction.
Embodiment 2
[0038] The preparation of embodiment 2N-(4-isobutoxyphenyl)-1H-imidazole-1-carboxamide (intermediate 2)
[0039] According to the method of Example 1, after the reaction, the reaction solution was concentrated under reduced pressure to constant weight to obtain N-(4-isobutoxyphenyl)-1H-imidazole-1-carboxamide.
Embodiment 3
[0040] The preparation of embodiment 3 pimavanserin
[0041] Add 124g (0.558mol) of N-(4-fluorophenyl)-1-methylpiperidin-4-amine in 500ml of toluene solution dropwise to N-(4-isobutoxyphenyl)-1H-imidazole- In the toluene solution of 1-formamide (prepared in Example 1), the temperature was raised and refluxed for 5 to 8 hours. After the reaction was completed, the temperature was lowered to room temperature, and 2.5 L of ethyl acetate was added to the reaction system, and washed twice with 1 L of saturated brine. Dry over anhydrous sodium sulfate. Suction filtration, adding 10 g of activated carbon to the filtrate, stirring at 80-85°C for 0.5-1 hour for decolorization, cooling to room temperature, suction filtration, evaporation under reduced pressure at 40°C to remove the solvent to obtain crude pimavanserin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com