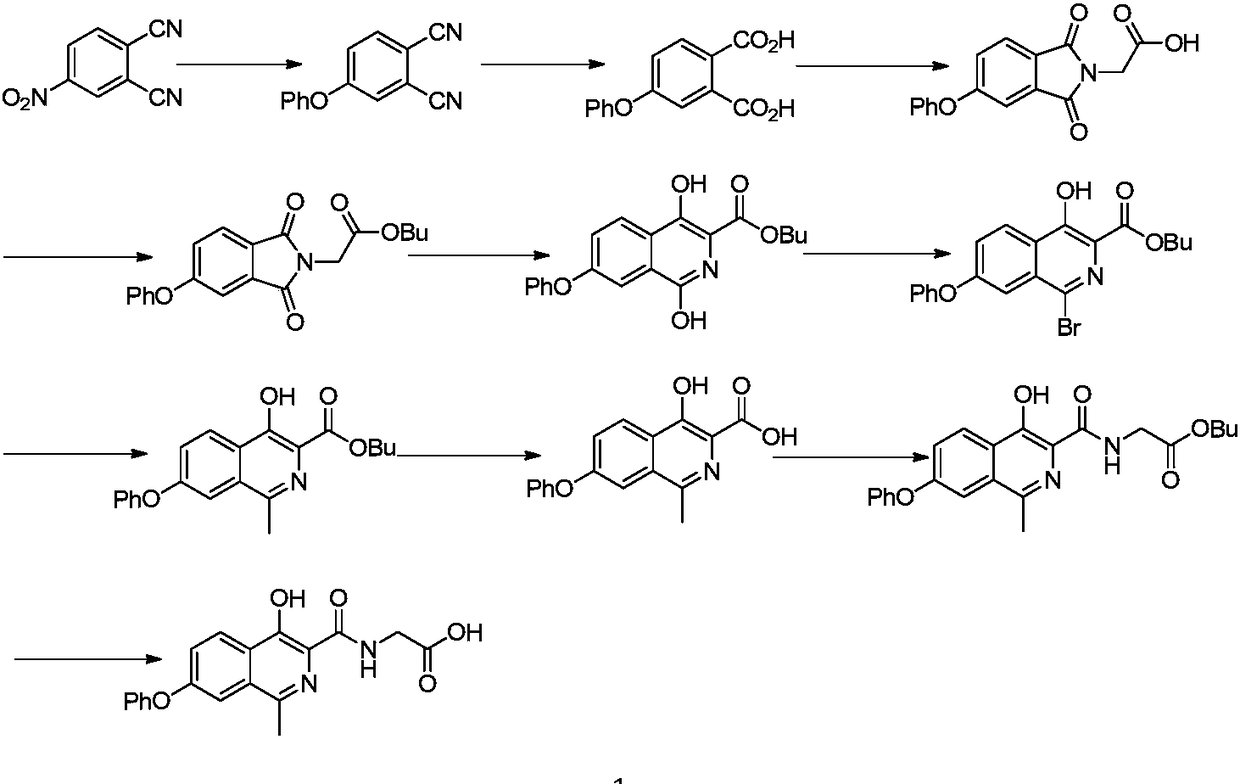
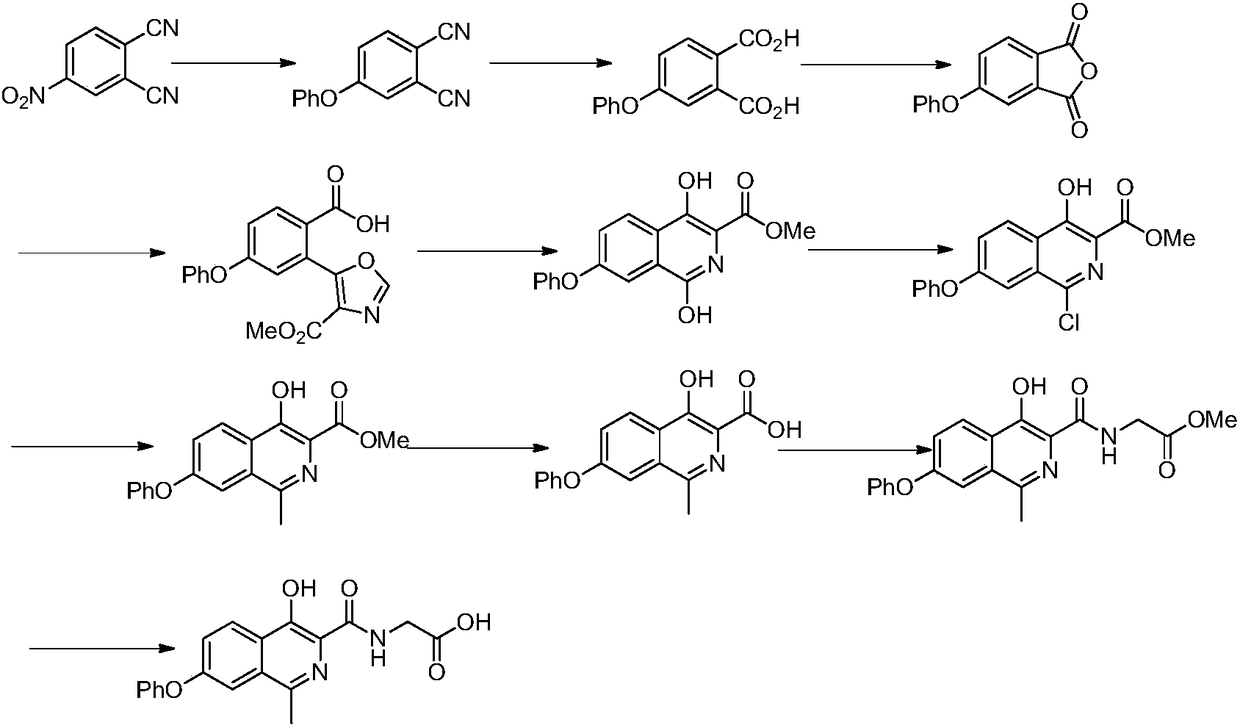
Preparation method of drug for chronic anemia
A synthetic method and compound technology, which is applied in the field of medicine and chemical industry, can solve the problems of difficult process amplification, long overall route, and low total yield, and achieve the effects of high product purity, reduced process cost, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047]Add compound 1a (27.23g, 100mmol) and dichloromethane (136mL) into reaction flask A, stir and dissolve, slowly drop into thionyl chloride (17.85g, 150mmol), reflux reaction for 4-6 hours, and distill under reduced pressure to When no distillate flowed out, dichloromethane (136 mL) was added and vortexed twice, and tetrahydrofuran (136 mL) was added to dissolve for later use. Add compound formula 2a (27.99g, 120mmol), anhydrous magnesium chloride (11.43g, 120mmol) and tetrahydrofuran (120mL) into the three-necked flask B, stir well, cool to -10~0°C in an ice-salt bath, add three Ethylamine (13.15 g, 130 mmol) was stirred at an internal temperature of -10 to 0°C for 60 minutes. Then slowly drop the acid chloride solution prepared in flask A into reaction flask B, and then slowly raise the temperature to 25-30°C to react for 6-8 hours. After the reaction, cool the reaction solution to 0-5°C, and slowly add saturated chlorine Ammonium chloride quenched the react...
Embodiment 2
[0049]
[0050] Add compound 1b (28.63g, 100mmol) and dichloromethane (143mL) into reaction flask A, stir and dissolve, slowly add thionyl chloride (17.85g, 150mmol) dropwise, reflux for 4-6 hours and then rotary evaporate to no Distillate flowed out, added dichloromethane (143mL) and vortexed twice, added acetonitrile (143mL) to dissolve and set aside. Add compound formula 2b (29.67g, 120mmol), anhydrous magnesium chloride (11.43g, 120mmol) and acetonitrile (120mL) into the three-necked flask B, stir evenly, cool to -10~0°C in an ice-salt bath, and add two Isopropylethylamine (16.80 g, 130 mmol) was stirred at an internal temperature of -10 to 0°C for 60 minutes. Then slowly drop the acid chloride solution prepared in flask A into reaction flask B, and then slowly raise the temperature to 25-30°C to react for 6-8 hours. After the reaction, cool the reaction solution to 0-5°C, and slowly add saturated chlorine Quench the reaction with ammonium chloride (143mL), add water (...
Embodiment 3
[0052]
[0053] Add compound 1c (30.03g, 100mmol) and dichloromethane (150mL) into reaction flask A, stir and dissolve, slowly add thionyl chloride (17.85g, 150mmol) dropwise, reflux reaction for 4 to 6 hours, and then rotary evaporate to no Distillate flows out, add dichloromethane (150mL) and swirl twice, add dichloromethane (150mL) to dissolve and set aside. Add compound formula 2b (29.67g, 120mmol), anhydrous magnesium chloride (11.43g, 120mmol) and dichloromethane (150mL) into the three-neck flask B, stir well, and cool to -10~0℃ in an ice-salt bath, under nitrogen protection Diisopropylethylamine (16.80 g, 130 mmol) was added, and the mixture was stirred for 60 minutes at an internal temperature of -10 to 0°C. Then slowly drop the acid chloride solution prepared in flask A into reaction flask B, and then slowly raise the temperature to 25-30°C to react for 6-8 hours. After the reaction, cool the reaction solution to 0-5°C, and slowly add saturated chlorine Ammonium c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com