Synthetic method for avibactam sodium salt
A kind of technology of avibactam sodium and synthesis method, applied in the field of synthesis of avibactam sodium, can solve problems such as weak sulfonation performance, long time, price increase cost, etc., to reduce production cost, shorten reaction time, improve The effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
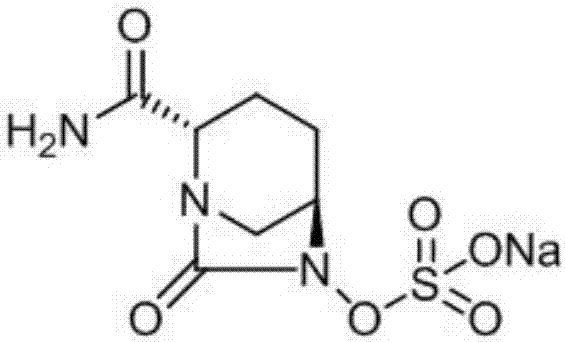
[0037] A kind of synthetic method of Avibactam sodium comprises the following steps:
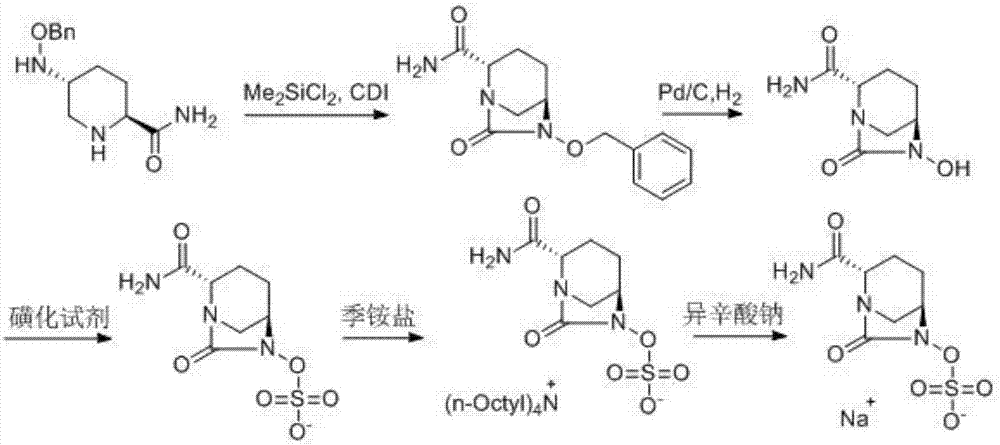
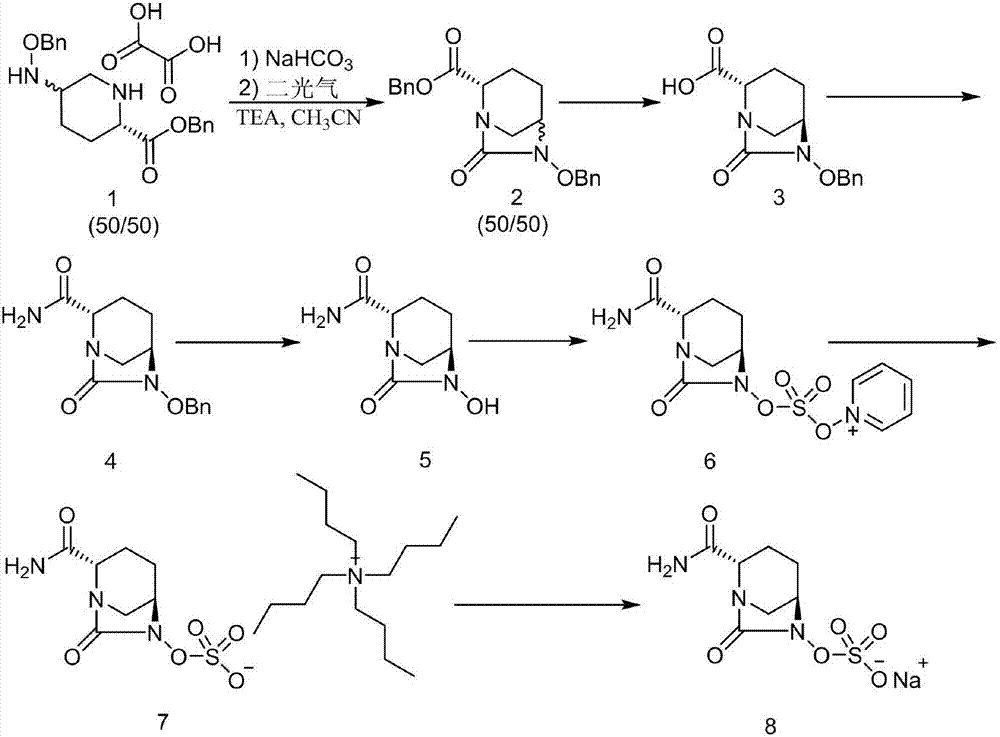
[0038](1) Measure acetonitrile (100mL) into the reaction flask, add (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide (10g), and control the temperature at 5-8°C , 20mL (3eq) N,N-diisopropylethylamine was added to the system, and then 6.4mL (1.3eq) dimethyldichlorosilane was added dropwise, and the reaction was complete as detected by TLC. Add 8.5g CDI (1.3eq) to the system, raise the temperature to 45°C and stir. After the reaction is complete as detected by TLC, add 9.2mL (3eq) isopropanol, and continue stirring at 45°C. Cool to room temperature, add 50mL toluene to the system, then add 140mL 2mol / L HCl solution, after the system is separated, collect the organic phase, concentrate to obtain a light yellow solid, wash with methyl tert-butyl ether to obtain (2S, 5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (9.8 g, yield 90%).
[0039] (2) Measure 50 mL each of is...
Embodiment 2
[0044] A kind of synthetic method of Avibactam sodium comprises the following steps:
[0045] (1) Measure acetonitrile (100mL) into the reaction flask, add (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide (10g), and control the temperature at 5-8°C , 20mL (3eq) N,N-diisopropylethylamine was added to the system, and then 6.4mL (1.3eq) dimethyldichlorosilane was added dropwise, and the reaction was complete as detected by TLC. Add 8.5g CDI (1.3eq) to the system, raise the temperature to 45°C and stir. After the reaction is complete as detected by TLC, add 9.2mL (3eq) isopropanol, and continue stirring at 45°C. Cool to room temperature, add 50mL toluene to the system, then add 140mL 2mol / L HCl solution, after the system is separated, collect the organic phase, concentrate to obtain a light yellow solid, wash with methyl tert-butyl ether to obtain (2S, 5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (9.8 g, yield 90%).
[0046] (2) Measure 50 mL each of i...
Embodiment 3
[0051] A kind of synthetic method of Avibactam sodium comprises the following steps:
[0052] (1) Measure acetonitrile (100mL) into the reaction flask, add (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide (10g), and control the temperature at 5-8°C , 20mL (3eq) N,N-diisopropylethylamine was added to the system, and then 6.4mL (1.3eq) dimethyldichlorosilane was added dropwise, and the reaction was complete as detected by TLC. Add 8.5g CDI (1.3eq) to the system, raise the temperature to 45°C and stir. After the reaction is complete as detected by TLC, add 9.2mL (3eq) isopropanol, and continue stirring at 45°C. Cool to room temperature, add 50mL toluene to the system, then add 140mL 2mol / L HCl solution, after the system is separated, collect the organic phase, concentrate to obtain a light yellow solid, wash with methyl tert-butyl ether to obtain (2S, 5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (9.8 g, yield 90%).
[0053] (2) Measure 50 mL each of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com