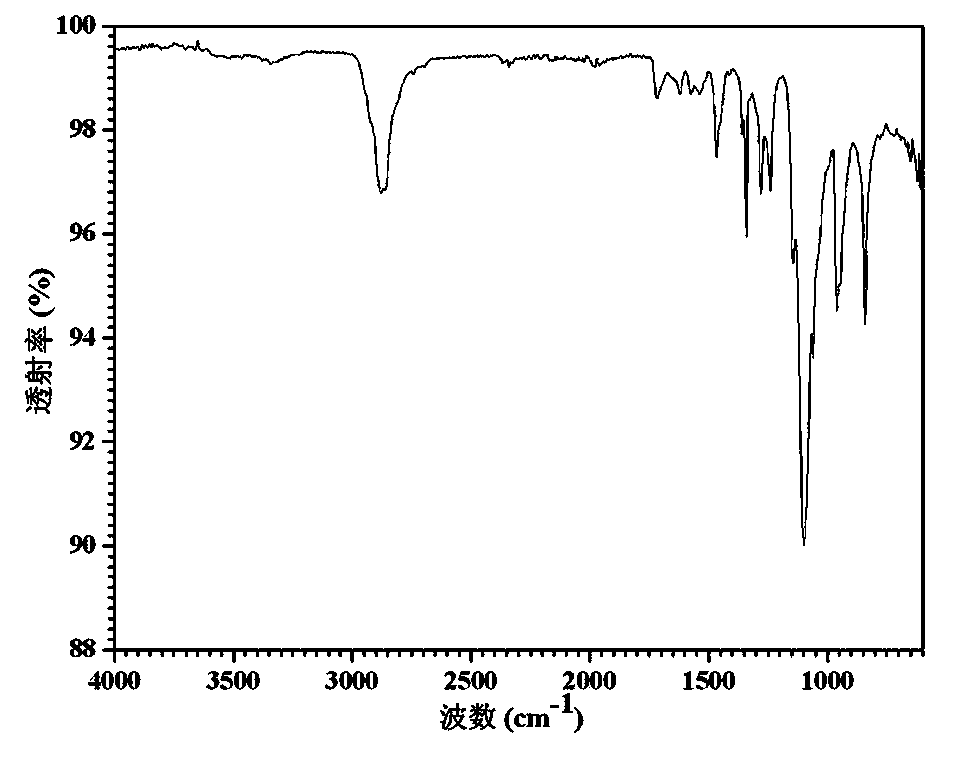
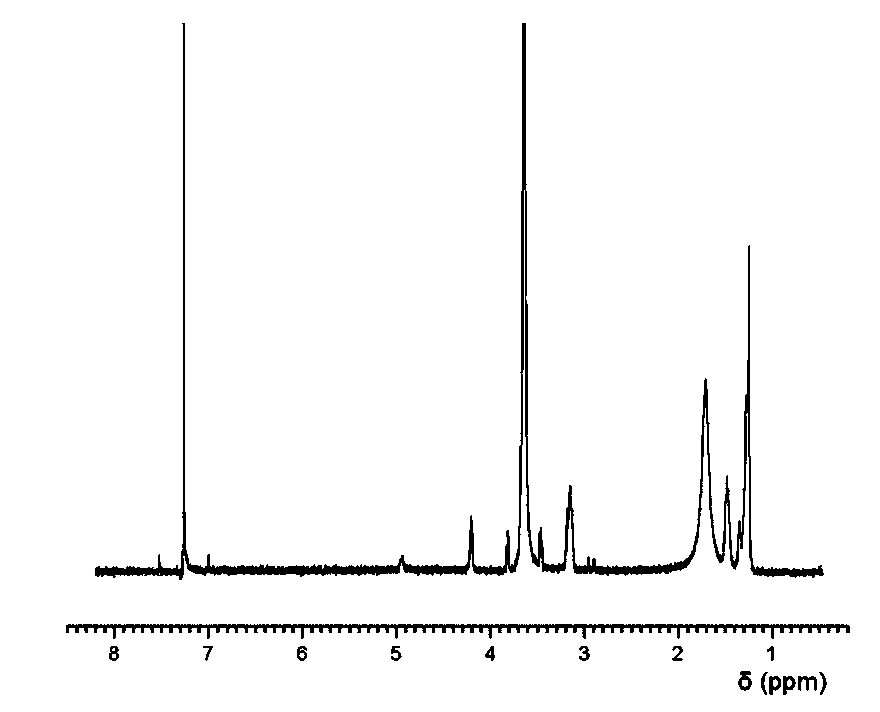
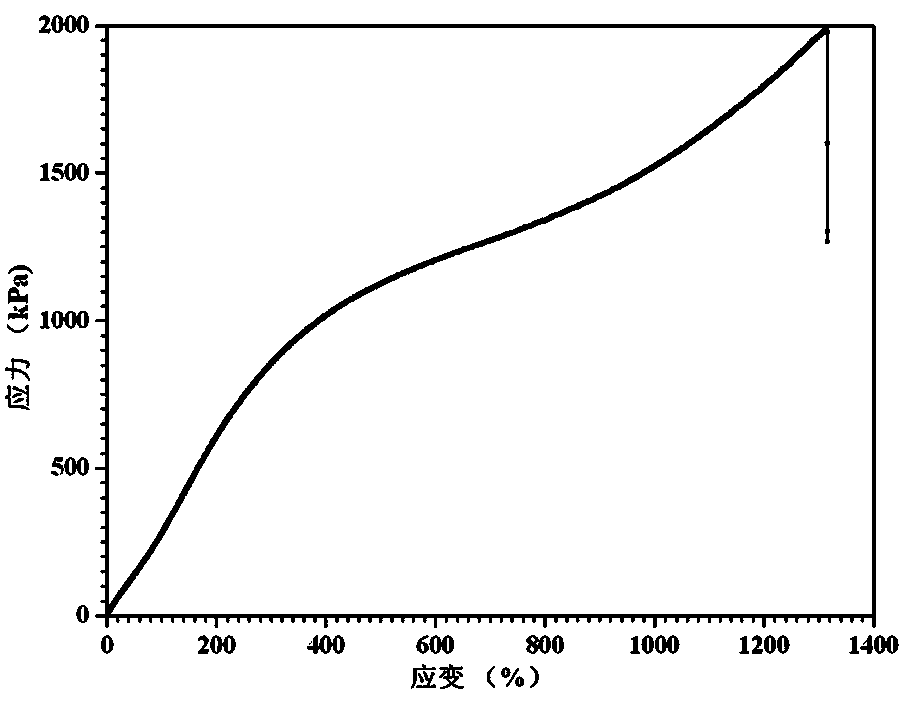
Polyurethaneurea hydrogel and preparation methods therefor
A technology of polyurethane urea and hydrogel, which is applied in the field of biomedical polymer materials and supramolecular chemistry, can solve problems such as failure, reduced activity of bioactive components, tissue damage, etc., and achieve the effect of convenient processing and application and providing mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of high-strength polyurethane urea hydrogel provided by the present embodiment, its synthetic route is:
[0044] ;
[0045] In the formula, R represents the alkyl or aromatic group between the two isocyanate groups in the diisocyanate, R' represents the alkyl or aromatic group between the two amine groups in the diamine, Represents polyethylene glycol (PEG), its molecular weight is 200 ~ 20000 g / mol; n is the degree of polymerization.
[0046] The specific steps of synthesis are: adding diisocyanate OCN-(CH 2 ) 12 -NCO, then add 5 μl catalyst dibutyltin dilaurate, and react at 80 °C for 2 h. Then add diamine H to the reaction flask 2 N-(CH 2 ) 6 -NH 2 , and stirred for 3 h at 20 °C under nitrogen protection. Strictly control the molar ratio of the reaction raw materials, that is, PEG: diisocyanate: diamine = 1:2:1. After the reaction, the obtained solution with high viscosity was precipitated in n-hexane to obtain a white flocculent solid. The produc...
Embodiment 2
[0059] This embodiment provides a synthetic method of high-strength polyurethane urea hydrogel, and the specific steps are as follows:
[0060] Add diisocyanate IPDI to the PEG acetone solution with a molecular weight of 10000 g / mol, and then add 5 μl catalyst stannous octoate, and react at 50 °C for 3 h. Then, the diamine 3,3′-methylenediphenylamine was added into the reaction flask, and stirred under nitrogen protection at 40 °C for 3 h. Strictly control the molar ratio of the reaction raw materials, that is, PEG: diisocyanate: diamine = 1:2:1. After the reaction, the obtained solution with high viscosity was precipitated in ether to obtain a white flocculent solid. The product is dried and dissolved in chloroform, and then poured into a mold for molding. After the solvent is evaporated and dried, it is allowed to swell in distilled water until the swelling balance is reached, and a polyurethane urea hydrogel is obtained.
[0061] The characteristic of this example is to u...
Embodiment 3
[0063] The high-strength polyurethane urea hydrogel provided in this example. The concrete steps of its synthetic method are as follows:
[0064] Add the diisocyanate 1,3-diisocyanate to the PEG acetone solution with a molecular weight of 200 g / mol, and then add 5 μl catalyst pyridine, and react at 80 °C for 3 h. Then add diamine to the reaction flask , stirred and reacted under nitrogen atmosphere at 40 °C for 3 h. Strictly control the molar ratio of the reaction raw materials, that is, PEG: diisocyanate: diamine = 1:2:1. After the reaction, the obtained solution with high viscosity was precipitated in n-hexane to obtain a white flocculent solid. The product is dried and dissolved in methanol, and then poured into a mold for molding. After the solvent is evaporated and dried, it is allowed to swell in distilled water until the swelling balance is reached, and a polyurethane urea hydrogel is obtained.
[0065] The characteristic of this embodiment is to use aromatic diisocy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com