Biodegradable hydrogel with temperature sensitivity and production method and use thereof
A hydrogel and biological technology, which is applied in the fields of polymer chemistry and biology, can solve the problems of non-degradability, etc., and achieve the effect of simple preparation method and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
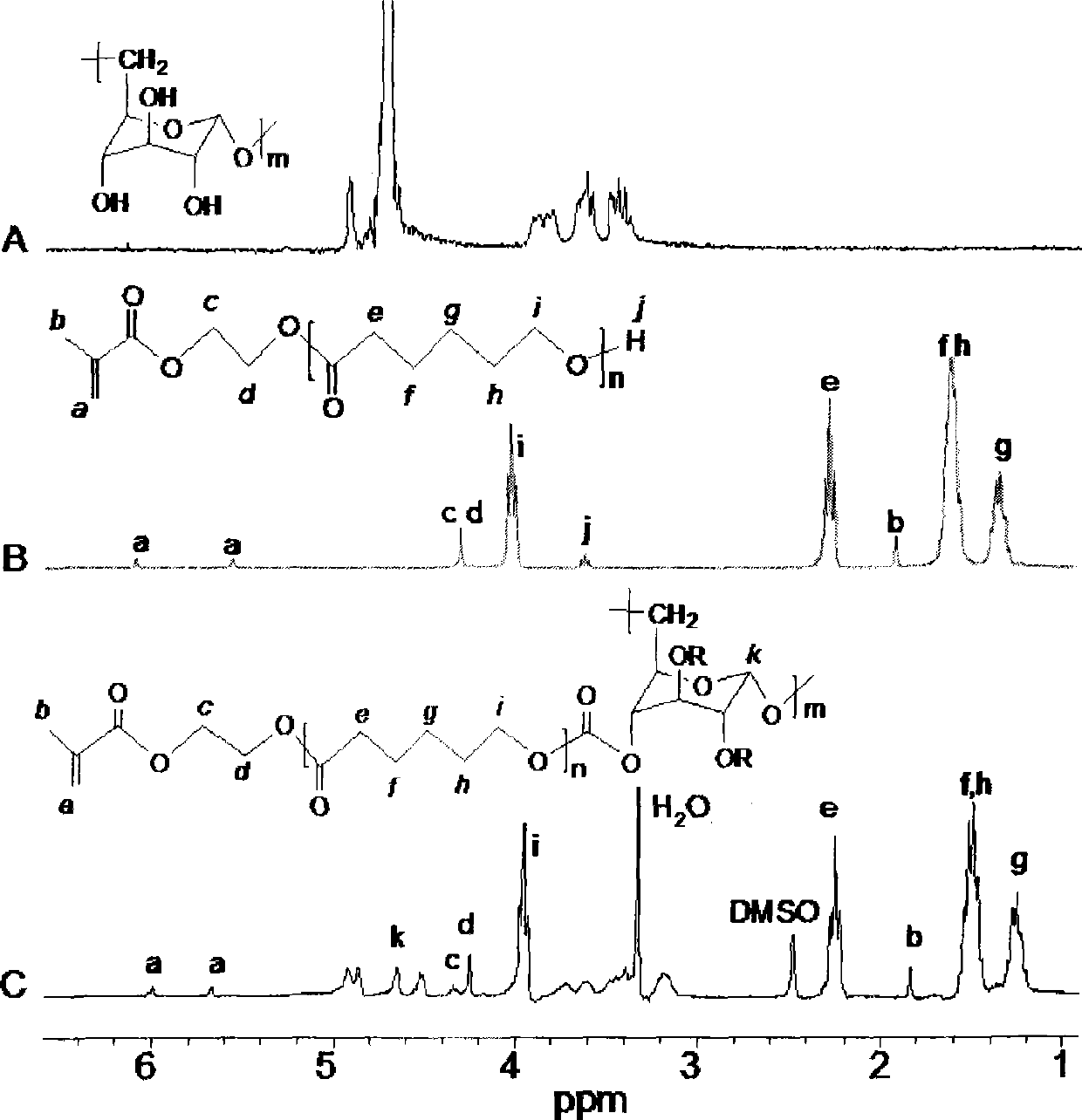
[0020] Embodiment 1: (1) Preparation of hydroxyethyl methacrylate-polycaprolactone—g-α-(1→6)-D-glucan: a: the hydroxyethyl methacrylate of equimolar ratio (HEMA) and ε-caprolactone (CL) in a nitrogen atmosphere, using stannous octoate as a catalyst, reacted at 110 degrees Celsius for 3 hours, wherein the molar ratio was hydroxyethyl methacrylate: ε-caprolactone: stannous octoate =1:1:0.01. The reacted mixture was dissolved in tetrahydrofuran, and then precipitated in cold water. The precipitate was dissolved in ethyl acetate, dried overnight with magnesium sulfate, and then concentrated under reduced pressure to obtain hydroxyethyl methacrylate-polycaprolactone (PCL-HEMA) . b: Dissolve the obtained product hydroxyethyl methacrylate-polycaprolactone and carbonyldiimidazole (CDI) in tetrahydrofuran at a molar ratio of 1:1, react in a nitrogen atmosphere for 24 hours, and obtain methacrylic acid by rotary evaporation Hydroxyethyl ester - polycaprolactone - imidazole (HEMA-PCL-C...
Embodiment 2
[0022] Embodiment 2: hydrogel controlled release bovine serum albumin (BSA) in vitro
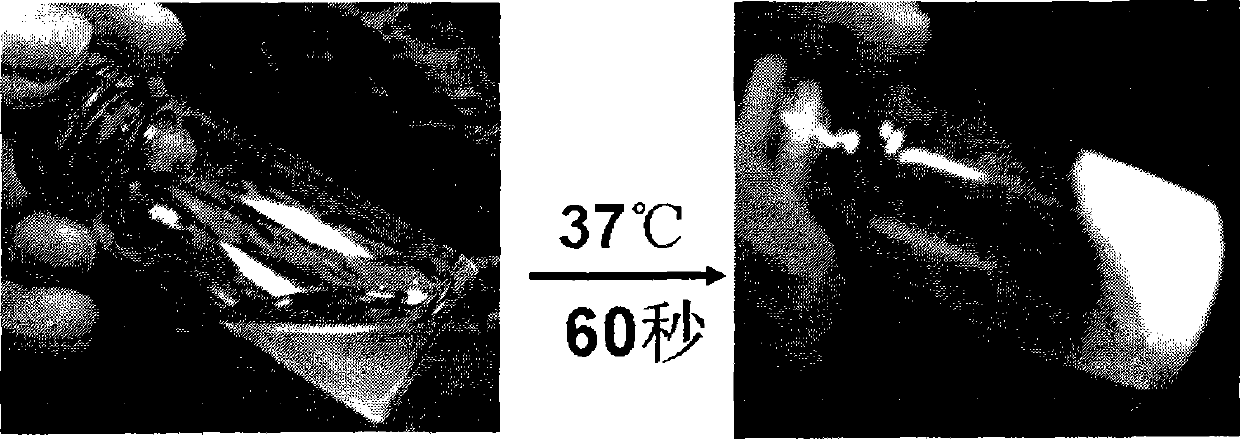
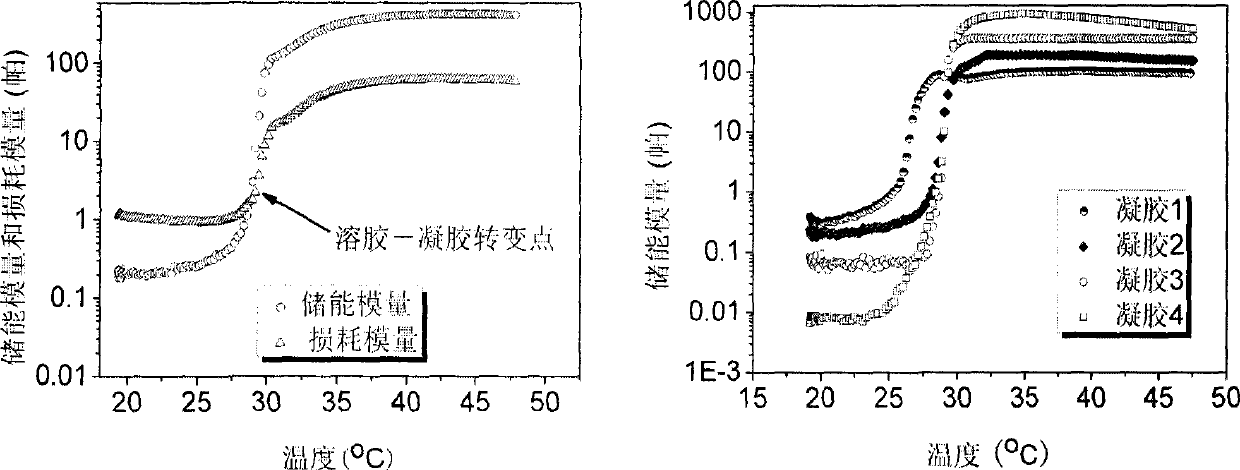
[0023] 20 mg of bovine serum albumin (number average molecular weight: 67,000) was dissolved in 1000 mg of DN2 hydrogel in a phosphate buffer solution, wherein the content of the hydrogel was 8%. Then, the phosphate buffer solution of this hydrogel was fully mixed with bovine serum albumin, placed in a 50 ml centrifuge tube overnight in a water bath at 37° C., so that the hydrogel solution became a viscous hydrogel. 20 ml of phosphate buffer solution warmed to 37° C. was slowly added to the DN2 hydrogel coated with bovine serum albumin. At the set time point, 4 milliliters of phosphate buffer solution was taken out from the 20 milliliter drug delivery system each time, and fresh phosphate buffer solution was added to keep the volume constant. The concentration of BSA therein is detected with an ultraviolet spectrometer, and the ultraviolet absorption wavelength is 280 nanometers, thereby ca...
Embodiment 3
[0024] Example 3: The hydrogel carries bone marrow mesenchymal stem cells and cardiomyocytes and injects them into rats, and compares the differentiation and function of bone marrow mesenchymal stem cells and the differentiation of cardiomyocytes in different environments.
[0025] Seven days after the rats were purchased, they were randomly divided into four groups: 1. myocardial infarction group (8 rats), 2. hydrogel injection group (8 rats), 3. cell injection group (8 rats), 4. injection of Cell and hydrogel groups (11 mice). All injections were made through a 29-gauge needle into the inner portion of the myocardium, and the volume of each injection was 200 microliters. The first group injected 200 microliters of DMEM / F12 medium into the inner layer of the myocardium; the second group injected 200 microliters of hydrogel; the third group injected 200 microliters of suspended 1*10 7 DMEM / F12 medium of bone marrow mesenchymal stem cells; the fourth group was injected with su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com