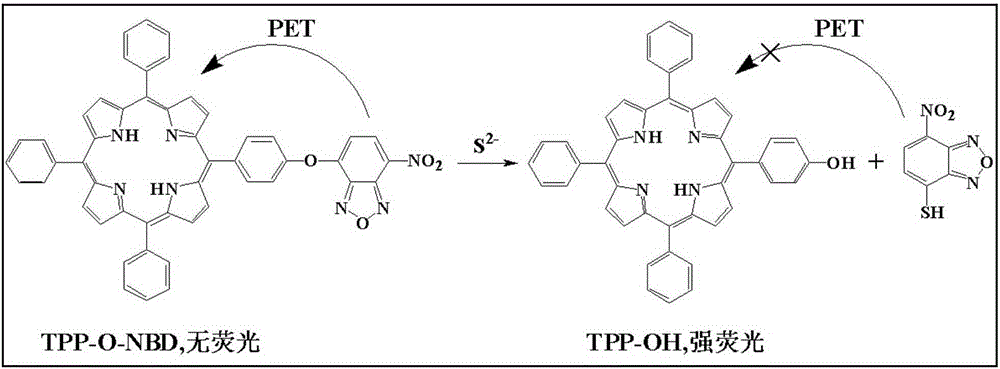
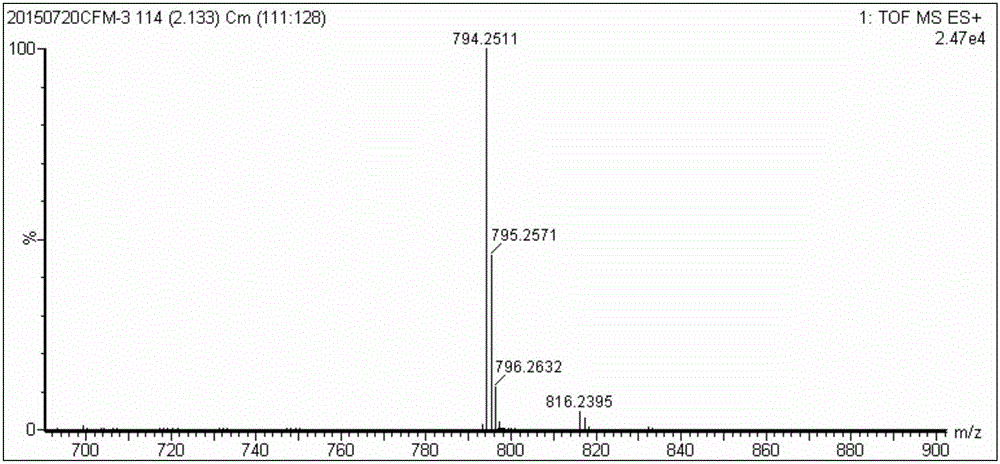
Preparation method and application of hydroxyl porphyrin-based high-selectivity near-infrared fluorescence sulfur ion probe
A high-selectivity, hydroxyporphyrin technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of simple synthesis route, easy purification, improved resolution and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method based on porphyrin-based highly selective near-infrared sulfur ion fluorescent probe (monohydroxyphenylporphyrin-7-nitrobenzofurazan), specifically comprising the following steps:
[0033] (1) Weigh monohydroxyphenylporphyrin (30mg, 0.048mmol) and DMAP (0.014ml, 0.116mmol) and dissolve them in 10ml refined dichloromethane to obtain solution A; then weigh 4-chloro-7-nitrobenzene Furazan (13.2 mg, 0.066 mmol) was dissolved in 10 ml of refined dichloromethane to obtain solution B. Fill solution A with N 2 After protecting for 20 to 30 minutes, slowly drop the solution of B into A with a constant pressure dropping funnel, and stir at room temperature for 24 to 36 hours to obtain monohydroxyphenylporphyrin-7-nitrobenzofurazan (TPP-O- NBD) in dichloromethane;
[0034](2) Wash the dichloromethane solution of TPP-O-NBD with saturated sodium chloride solution 3 to 4 times, each time with 15-20mL saturated sodium chloride solution, and then dry the organic ...
Embodiment 2
[0037] Sulfur ion detection experiment.
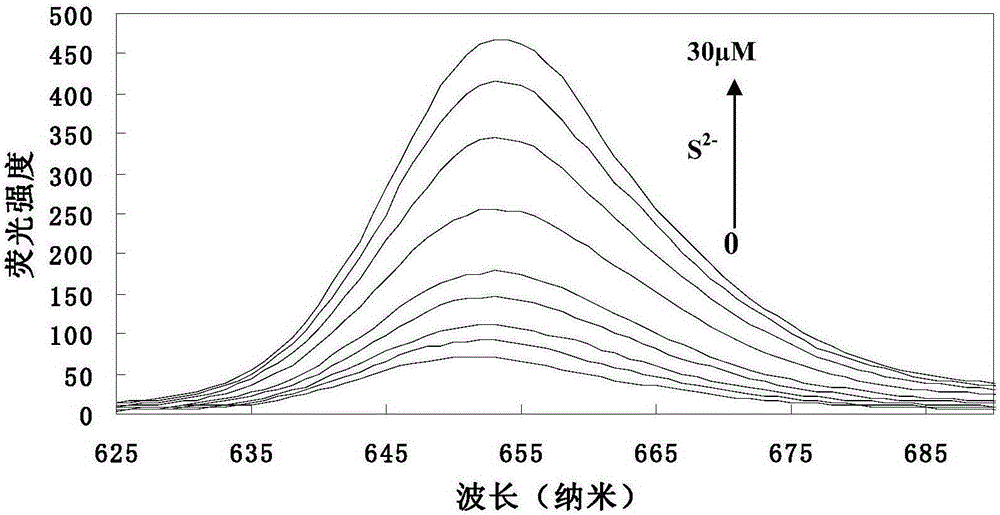
[0038] (1) Add 5.0ml of THF, 1.50mmol L to a 10ml volumetric flask -1 Probe solution 66.5 μl, pH 7.4 1.0mol.L -1 0.5ml of 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer solution was diluted to 10ml with twice distilled water to obtain a concentration of 10μmol L -1 probe solution. Take 3.0ml probe solution and add it into 4ml cuvette, add S 2- , so that the concentrations are 0, 1.0, 3.0, 5.0, 7.0, 9.0, 15.0, 20.0, 25.0, 30.0 μmol L -1 . Stir the reaction at room temperature for 5 minutes to measure its fluorescence emission spectrum (λ ex =428nm,λ em = 652nm). to obtain probes with different concentrations of S 2- The fluorescence emission spectrum of the reaction (see image 3 ). Depend on image 3 It can be seen that the fluorescence intensity of the probe solution itself is very small, when adding S 2- After 5 minutes of reaction, the fluorescence intensity of the reaction system was significantly enhanced, ...
Embodiment 3
[0041] Sulfide ion selectivity experiment.
[0042] (1) Add 5.0mlTHF, 1.50mmol.L, to a 10ml volumetric flask -1 Probe solution 66.5 μl, pH 7.4 1.0mol.L -1 0.5ml of HEPES buffer solution, dilute to 10ml with twice distilled water, and obtain a concentration of 10μmol L -1 Probe solution. Take 3.0ml probe solution and add it to 4ml cuvette, add anion or related species respectively: S 2- , I - , Cl - , Br - ,C 2 o 4 2- , SO 3 2- , HSO 3 - , F - ,NO 2 - , SO4 2- ,NO 3 - , S 2 o 3 2- , CO 3 - , SCN - , HCO 3 - , Cys, Asp, H 2 o 2 , ClO - , GSH. Make S 2- The concentration is 30μmol.L -1 , the concentration of other ions and related species is 300 μmol.L -1 , after stirring at room temperature for 5 minutes, measure its fluorescence intensity (λ ex =428nm), get the fluorescence emission spectrum after the probe reacts with different anions or related species (see Figure 5 ). Depend on Figure 5 It can be seen that adding S 2- After adding othe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com