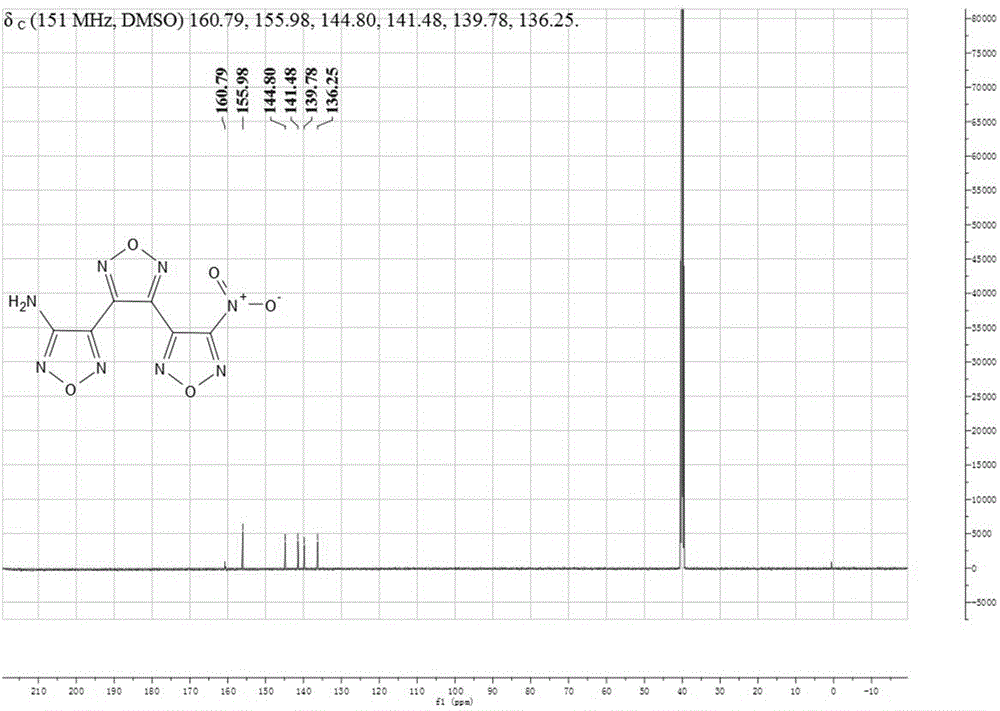
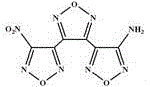
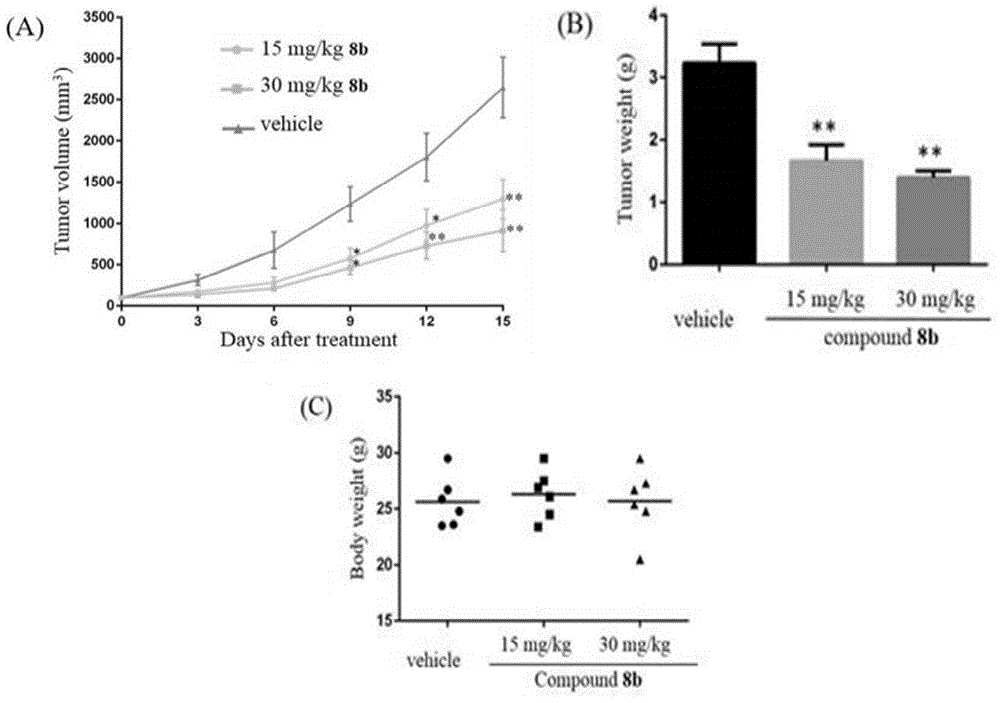
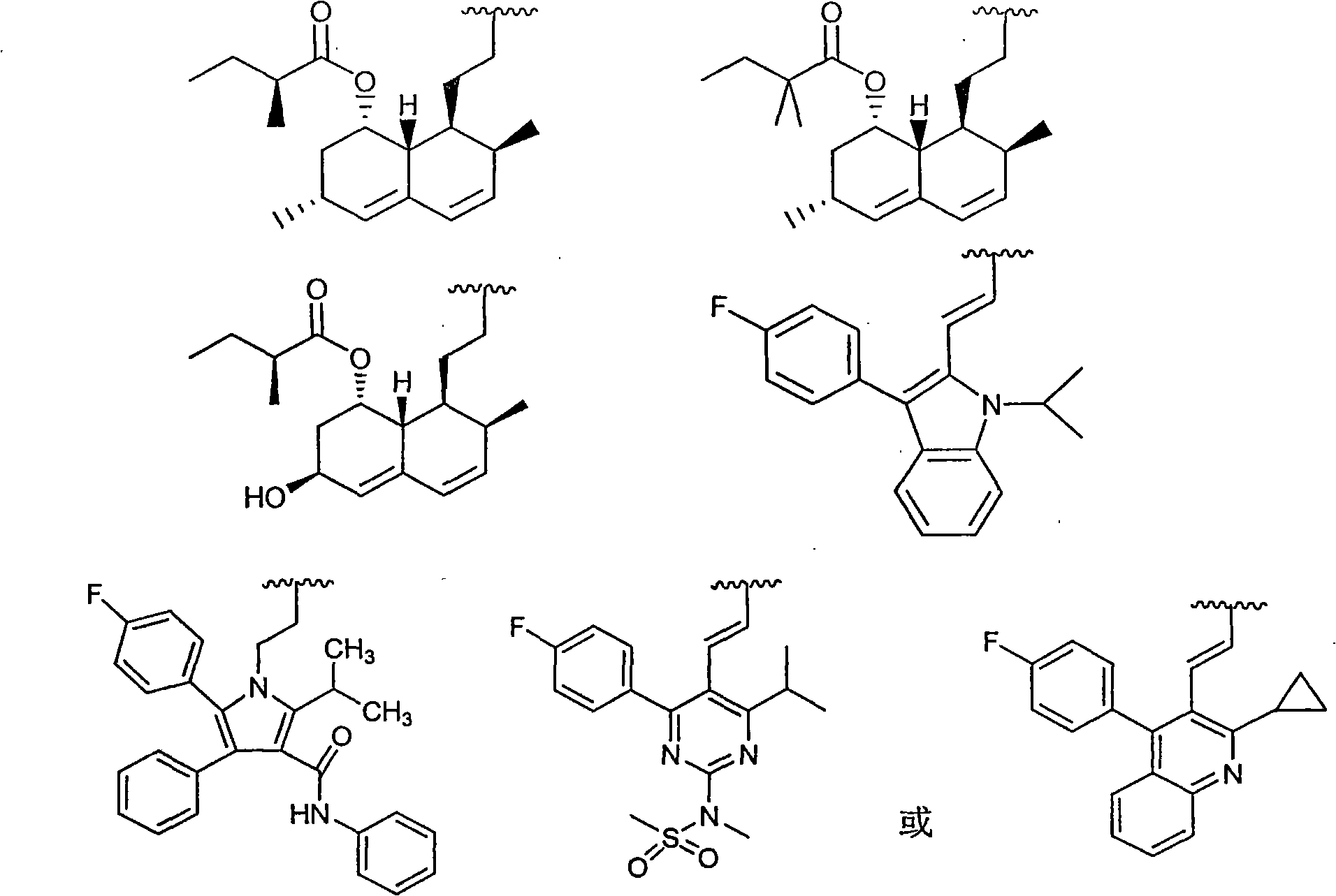
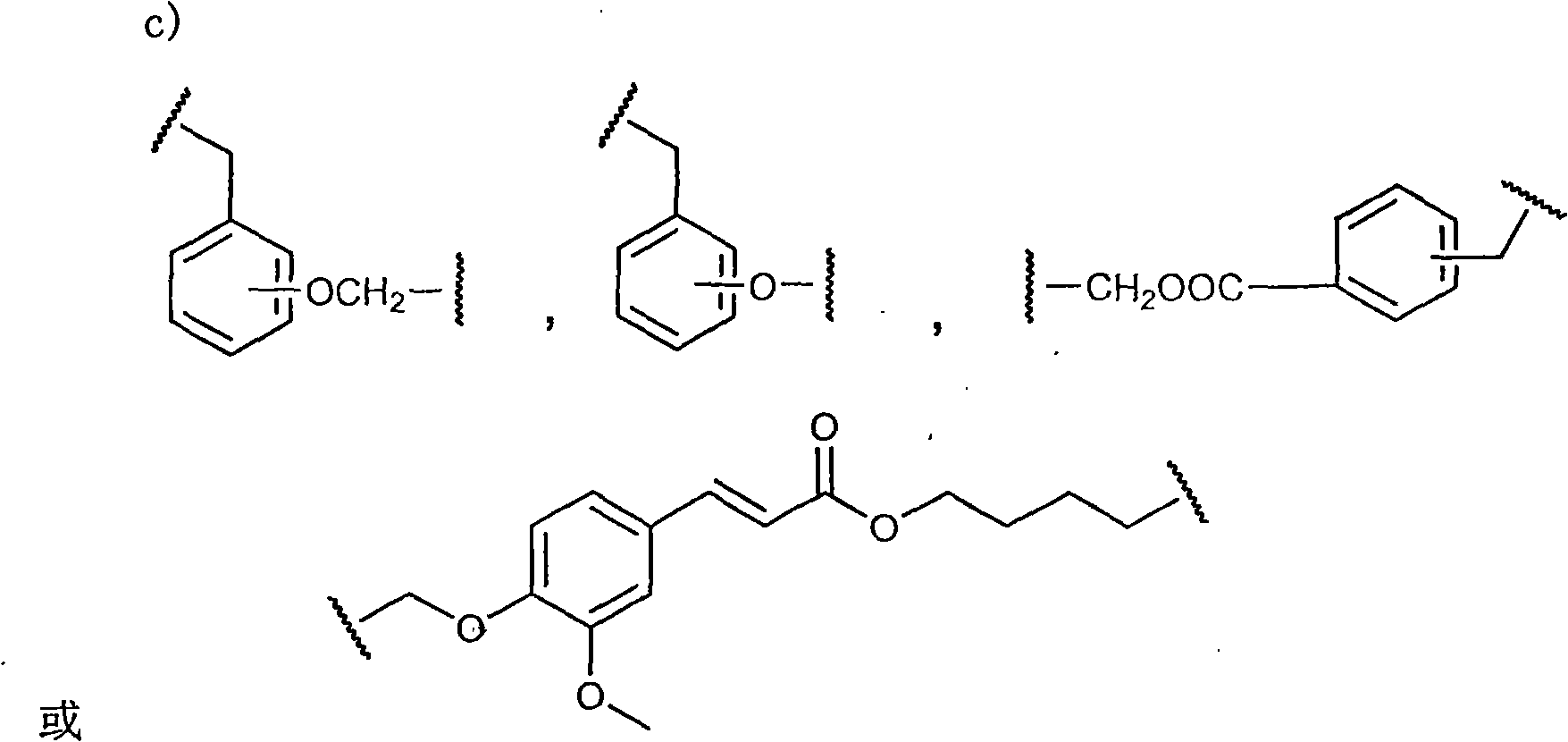
Patents
Literature
90 results about "Furazan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Furazan, or 1,2,5-oxadiazole, is a heterocyclic aromatic organic compound consisting of a five-atom ring containing 1 oxygen and 2 nitrogen atoms. The furazan ring system is also found in the steroid furazabol. Furazan and its derivatives are obtained from the oxime derivatives of 1,2-diketones.
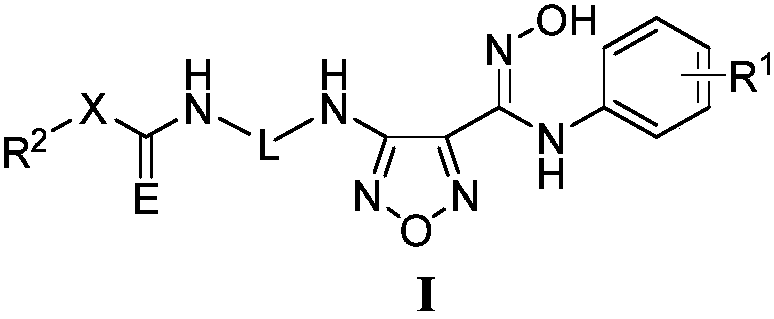
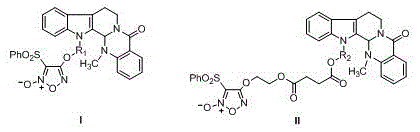
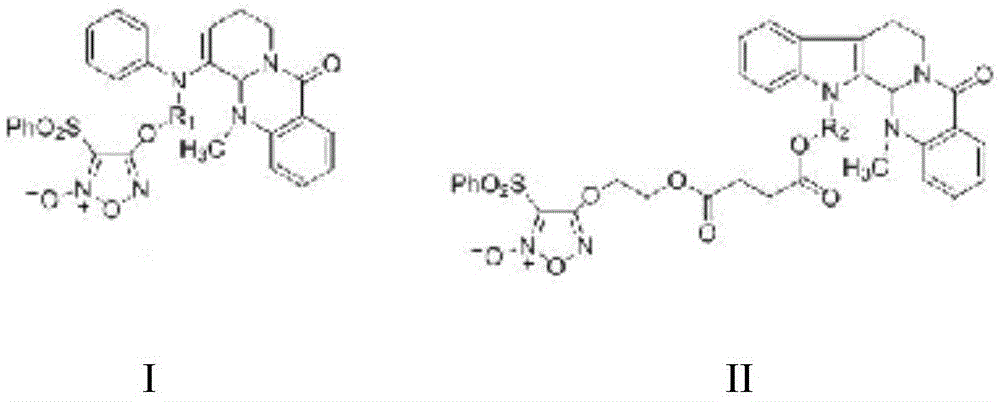
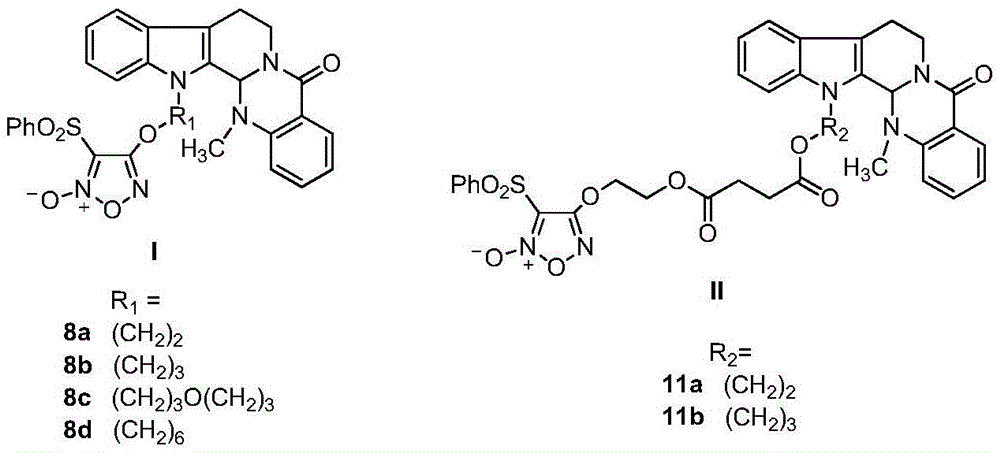
Furazan NO donor type evodiamine derivatives with anti-tumor activity
The invention relates to the field of natural medicines and medicinal chemistry and particularly relates to derivatives with modified 13-N of evodiamine. The invention discloses a preparation method of the 13-N furazan NO donor substituted evodiamine derivatives and evaluation of the anti-tumor activity. The structure of the compounds is shown in the specification, wherein R1 and R2 are (CH2)n or (CH2)n1O(CH2)n2, and n, n1 and n2 are integers between 1 and 8.
Owner:SHENYANG PHARMA UNIVERSITY
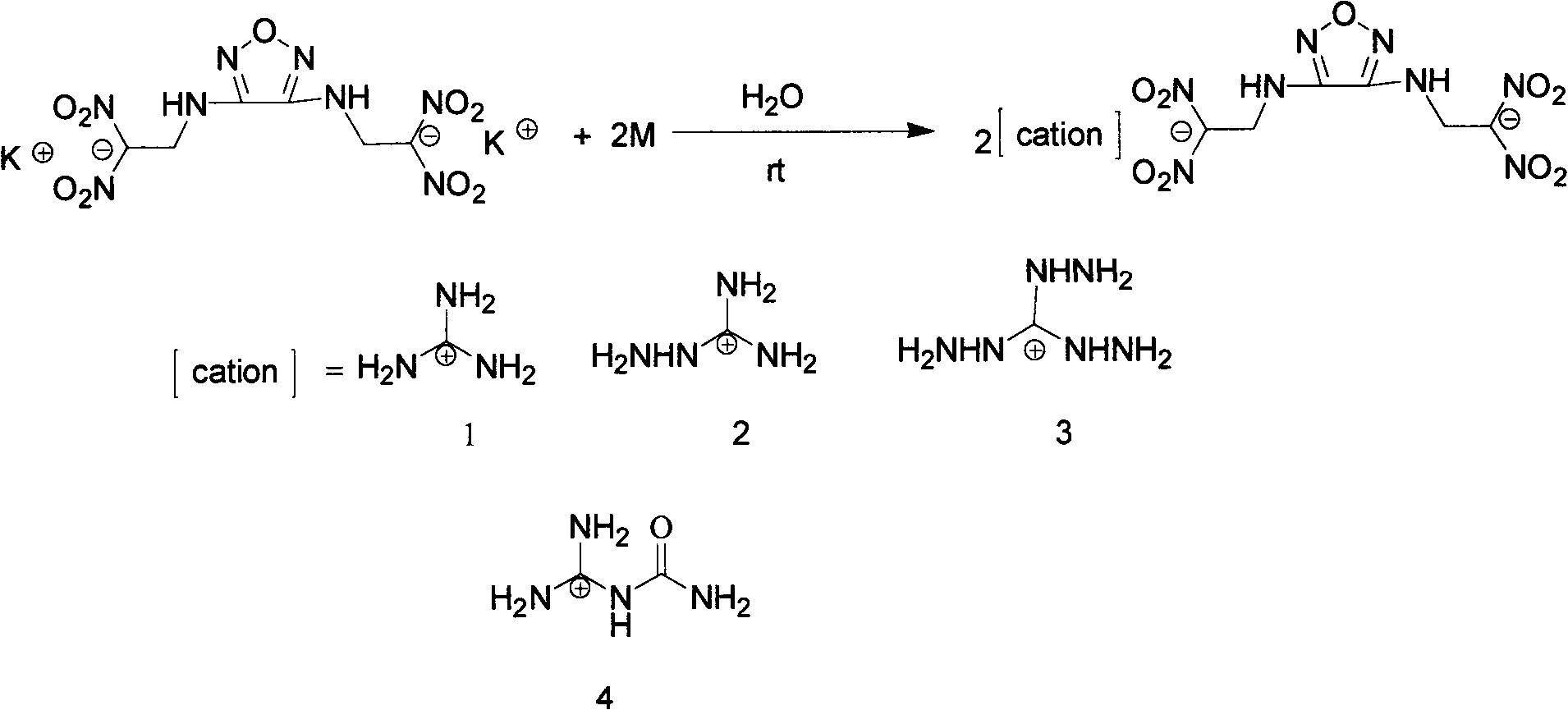
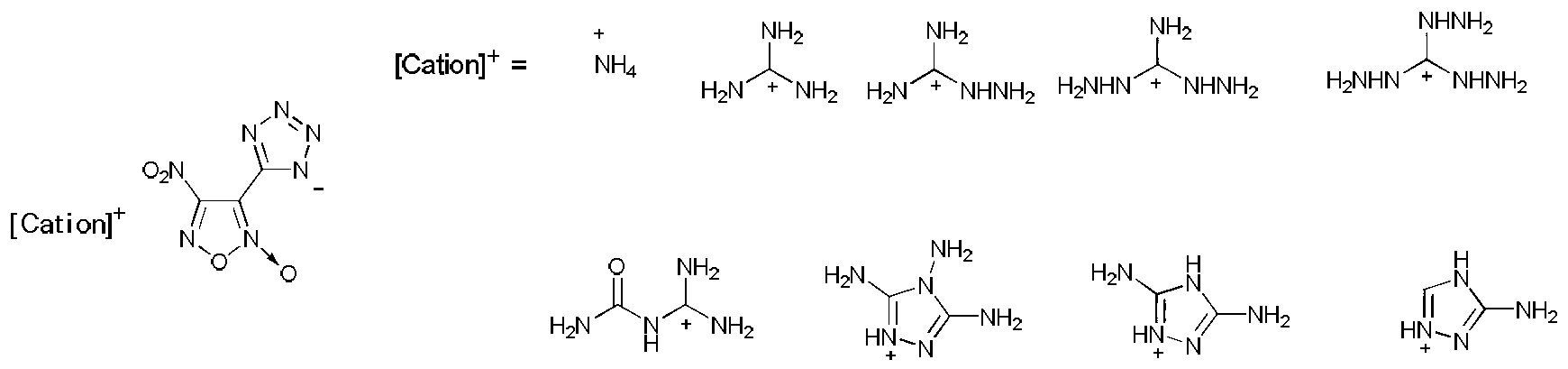
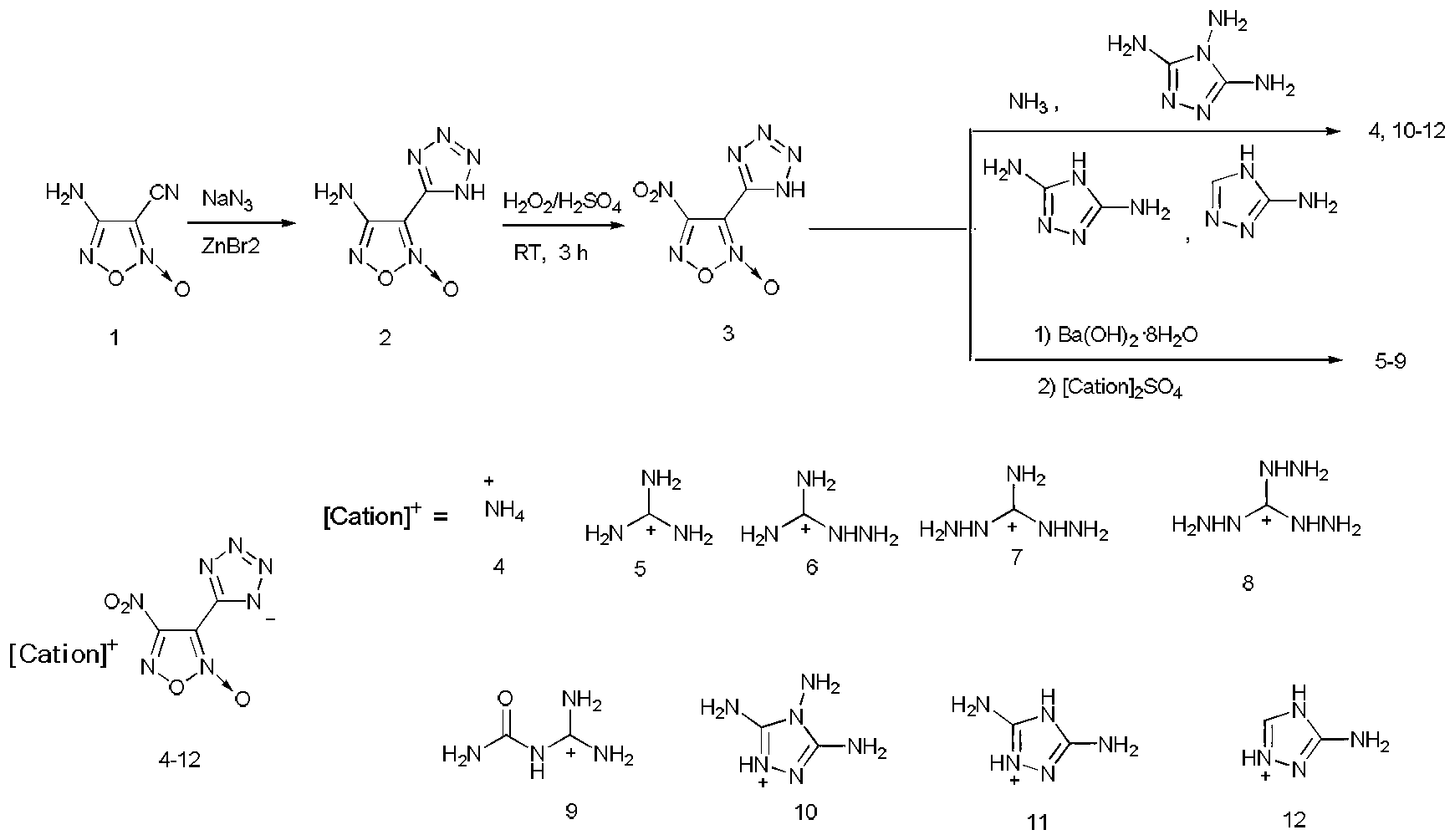
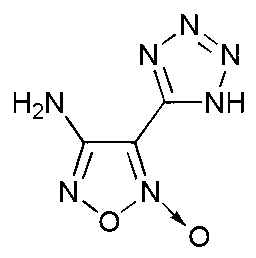
4-nitro-3-(5-tetrazole) furoxan energetic ionic salt and preparation method thereof
InactiveCN103059009AThe synthesis method is simpleMild conditionsOrganic chemistryOrganic compound preparationDetonationHigh density
The invention discloses a 4-nitro-3-(5-tetrazole) furoxan energetic ionic salt and a preparation method thereof, belongs to the technical field of energetic materials. A synthesis method of the 4-nitro-3-(5-tetrazole) furoxan energetic ionic salt is as follows: directly reacting the 4-nitro-3-(5-tetrazole) furoxan with a corresponding cation, and steaming for eliminating a solvent so as to obtain a target product; reacting the 4-nitro-3-(5-tetrazole) furoxan with the sulfate of the equimolar corresponding cation after mixing the 4-nitro-3-(5-tetrazole) furoxan with equimolar Ba(OH)2.8H2O, filtering and precipitating, steaming and eliminating the solvent in the filtrate to obtain the target product. The synthesis method provided by the invention is simple and easy to industrialize. The referred 9 energetic ionic salt has high density (rho: 1.55-1.84g / cm3), wherein the degree of percussion sensitivity of two compounds is more than 40J, and the energetic ionic salt belongs to insensitive explosive. The energetic ionic salt has excellent calculation detonation property and is a potential energetic material.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Furazan derivative of coumarin parent nucleus and antineoplastic activity
ActiveCN105153142ANovel structureInhibitory activityOrganic active ingredientsOrganic chemistryHigh concentrationCancer cell
Owner:FUDAN UNIV
Statins antilipemic drugs furazan nitroxides derivates and preparation method thereof
The invention relates to a statins antilipemic drugs furazan nitroxides derivates and a preparation method thereof, and solves the problems of nonlipid-lowering and low activity in statins antilipemic drugs and adverse effect. The invention is shown as formula (1).
Owner:四川抗菌素工业研究所有限公司 +1
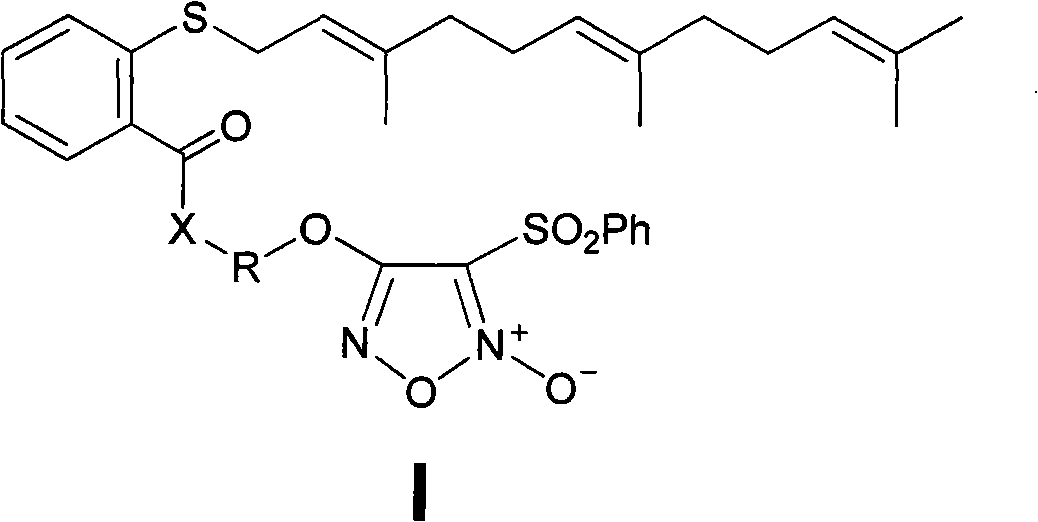
Nitric oxide donor-type farnesyl thiosalicylic acid derivative, and preparation method and medical application thereof
The invention discloses a nitric oxide (NO) donor-type farnesyl thiosalicylic acid (FTA) derivative, and pharmaceutically acceptable salt, a preparation method and medical application thereof. The FTA derivative is a compound obtained by carrying out heterozygosis on a NO donor furazan nitrogen oxide and Ras protein inhibitor FTA by an ester bond or an amido bond. Pharmacological test results show that the FTA derivative can reserve the Ras protein inhibiting activity of FTA and simultaneously releases high-concertration NO to induce cancer cell apoptosis and enhance the inhibiting action on cancer cell proliferation; compared with the FTA, the FTA derivative has more excellent anti-tumor activity, and therefore, the compound can be suitable for treating various clinical malignant tumours.
Owner:CHINA PHARM UNIV
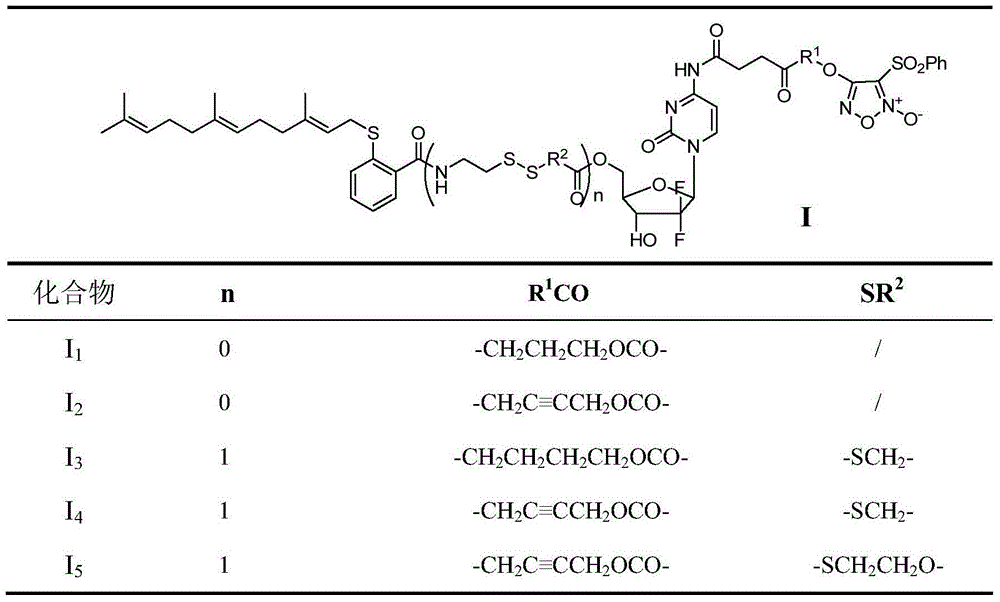
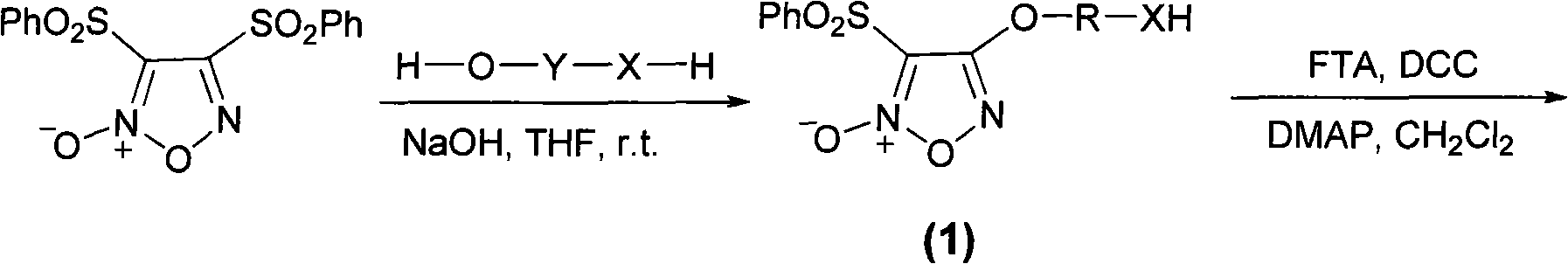
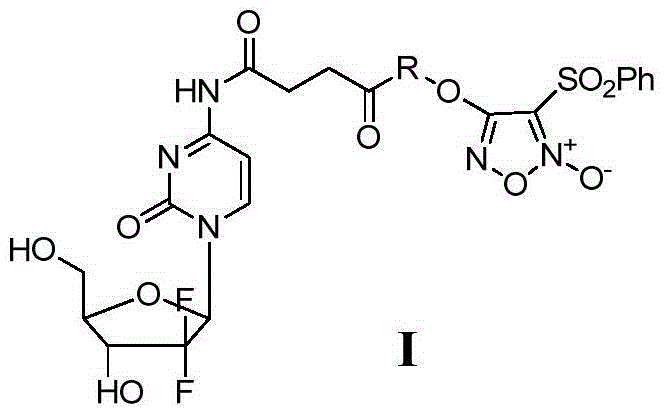
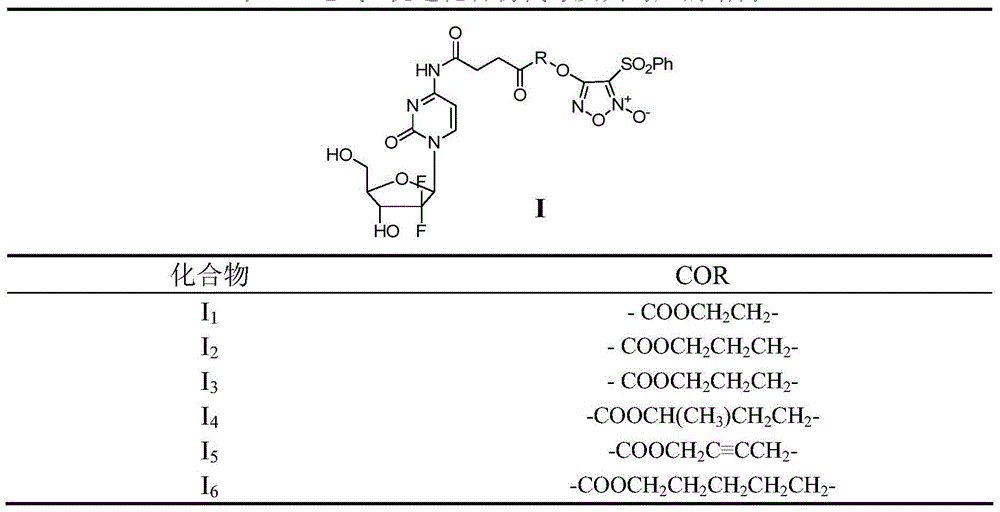
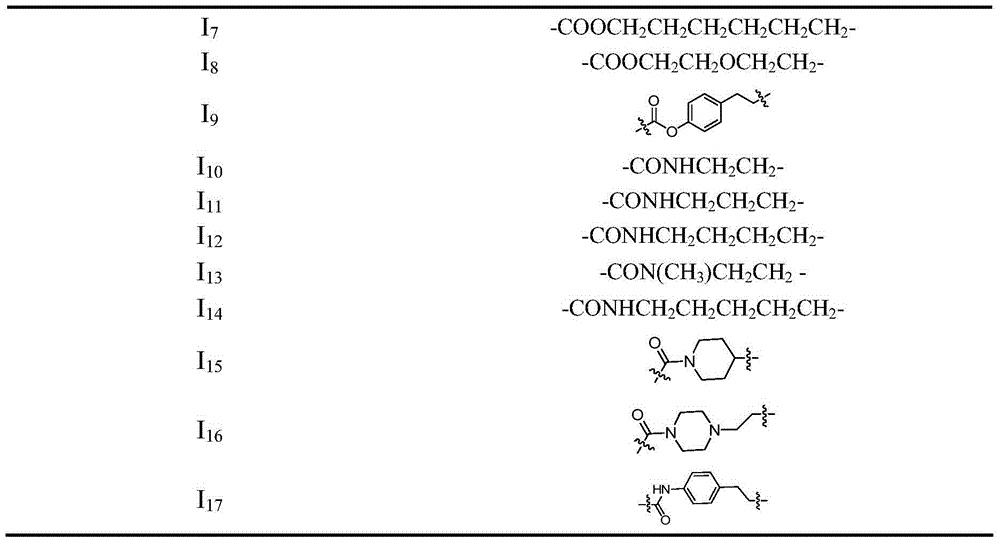
Benzenesulfonyl furazan modified gemcitabine derivative and preparation method and use thereof
The invention discloses a benzenesulfonyl furazan modified gemcitabine derivative and a preparation method and use thereof. The benzenesulfonyl furazan modified gemcitabine derivative comprises a compound as shown in a universal formula I and pharmaceutically acceptable salts thereof. According to the rational drug design principle and the combination principle, the clinical pharmacology activity of gemcitabine is retained; meanwhile, with gemcitabine as a lead compound, a benzenesulfonyl furazan NO donor is respectively coupled to N4 amino of gemcitabine through different linking groups to synthesize a benzenesulfonyl furazan modified NO donor type gemcitabine derivative, in order to obtain a compound with stronger anti-tumor activity and better bioavailability than gemcitabine.
Owner:苏州康纯医药科技有限公司
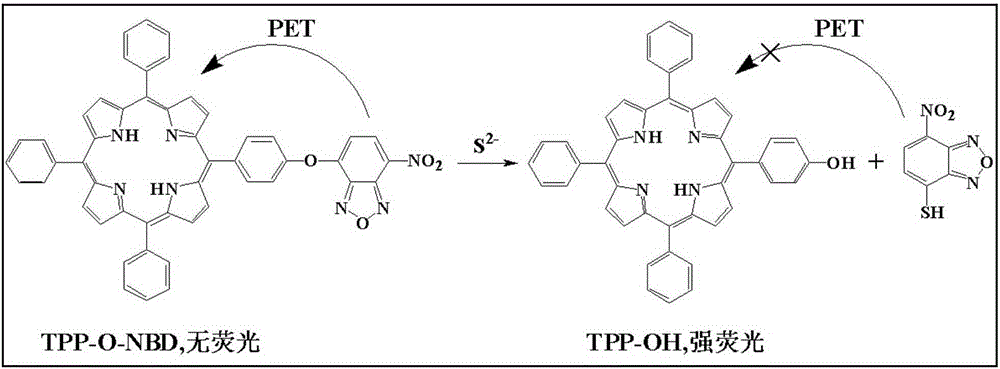
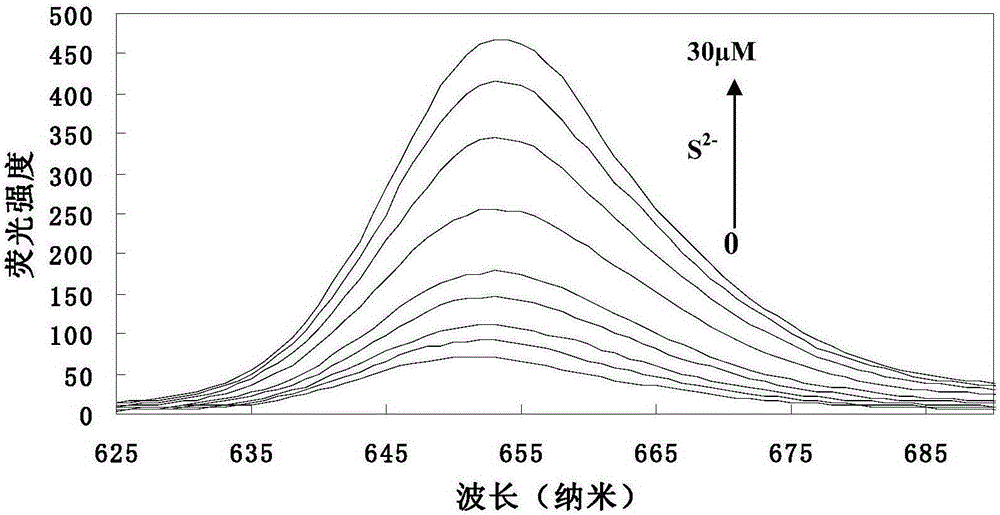
Preparation method and application of hydroxyl porphyrin-based high-selectivity near-infrared fluorescence sulfur ion probe
ActiveCN105295900AFast fluorescent responseHigh sensitivityOrganic chemistryFluorescence/phosphorescencePorphyrinHigh selectivity
The invention discloses a preparation method and application of a hydroxyl porphyrin-based high-selectivity near-infrared fluorescence sulfur ion probe. The fluorescence probe is prepared by taking 5-hydroxyphenyl-11,15,20-triphenylporphyrin (TPP-OH) and 4-chlorine-7-nitro-1,2,3-benzoxadiazole (NBD-Cl) as materials, taking 4-dimethylamino pyridine (DMAP) as a catalyst, taking dichloromethane as a solvent and performing one step reaction under room temperature. The preparation method of the probe is simple, and a product is easy to separate and purify. Compared with an existing small organic molecule fluorescence probe, the fluorescence probe obtained by the preparation method has higher fluorescence emission wavelength (lumbda em is greater than 650nm), high-sensitivity and high-selectivity quick recognition and detection on a trace of hydrogen sulphide can be realized in a near-infrared region, and the application prospect is great in the field of environmental and biological detection.
Owner:HUNAN UNIV OF SCI & TECH
Compound containing rhodamine groups and benzofurazan groups and preparation method and application thereof
InactiveCN104949949AImprove accuracyEasy to prepareFluorescence/phosphorescenceMercuric ionDiethylenetriamine
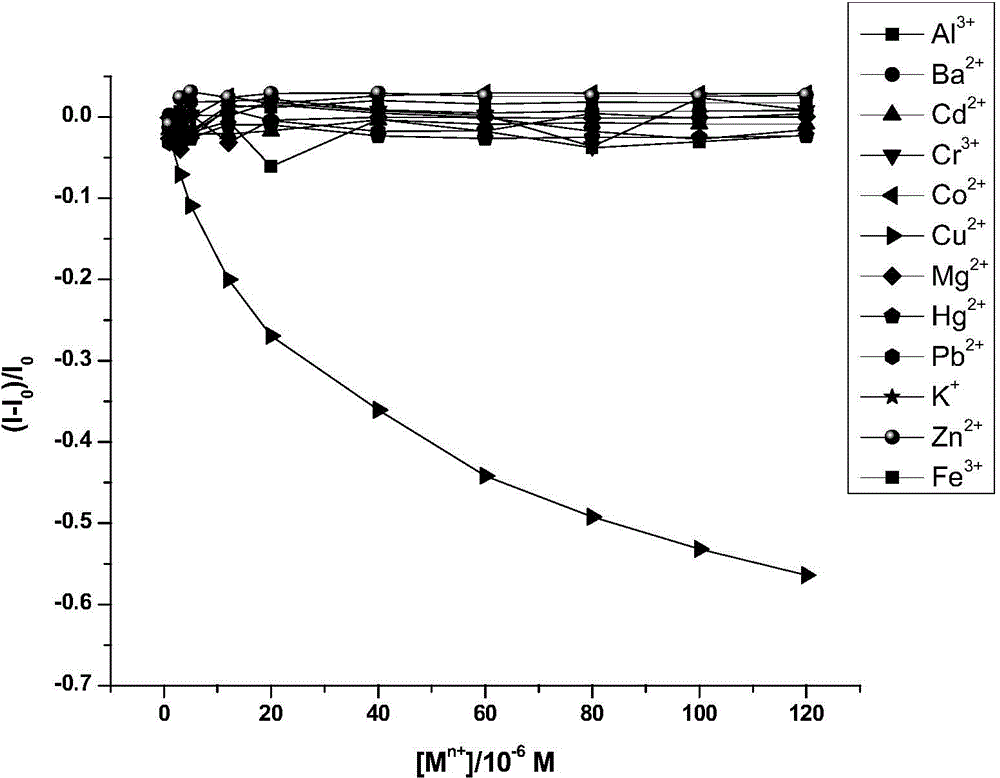
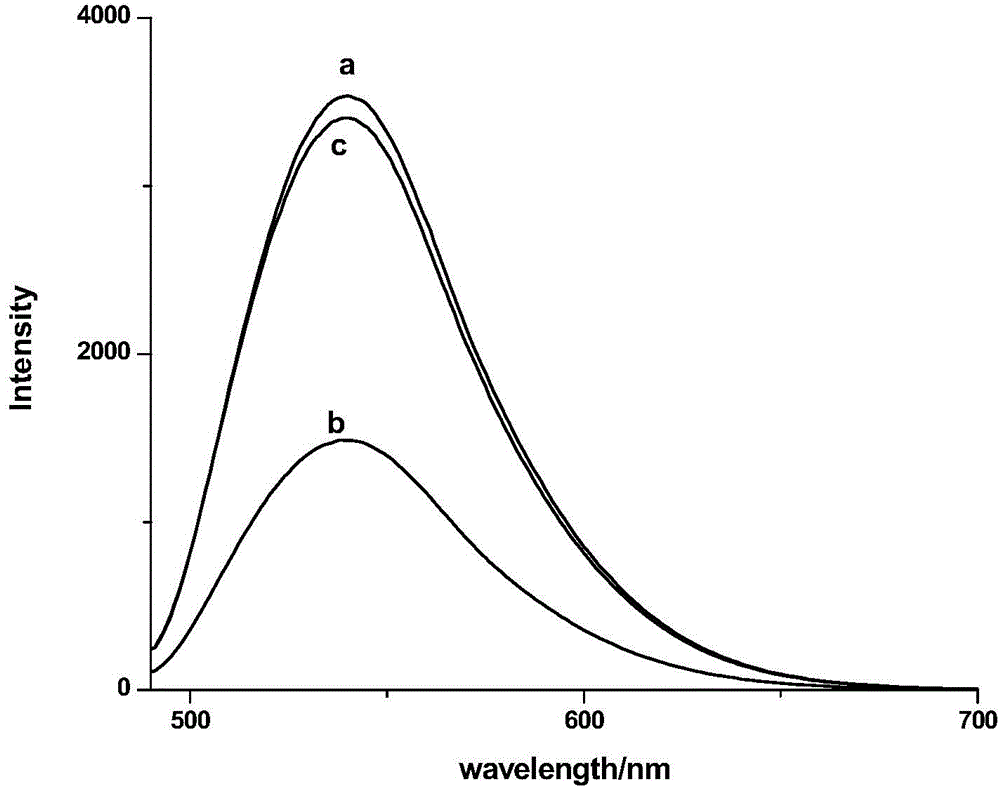
The invention discloses a compound containing rhodamine groups and benzofurazan groups and a preparation method and application thereof. The structural formula of the compound is as shown in the specification. The preparation method comprises the following steps: (1) enabling rhodamine B and diethylenetriamine to fully react in a solvent, removing the solvent, and performing preliminary separation and purification; (2) enabling rhodamine B acyl diethylenetriamine and 4-chloro-7-nitrobenzofuroxan to fully react in a solvent, monitoring the reaction process by virtue of TLC, and performing column elution after the reaction is ended, thereby obtaining the product. The method for detecting the concentration of mercury ions or iron ions comprises the following steps: (1) making a standard curve; (2) detecting and recording; and (3) calculating. The invention further discloses an application of the compound serving as an iron ion fluorescence probe or a mercury ion fluorescence probe. The invention also discloses an application of the compound serving as an iron ion specific fluorescence probe or a mercury ion specific fluorescence probe. The compound disclosed by the invention can serve as a specific fluorescence probe for mercury ions or iron ions, is used for detecting mercury ions or iron ions in the solution and is high in accuracy.
Owner:SOUTH CHINA NORMAL UNIVERSITY
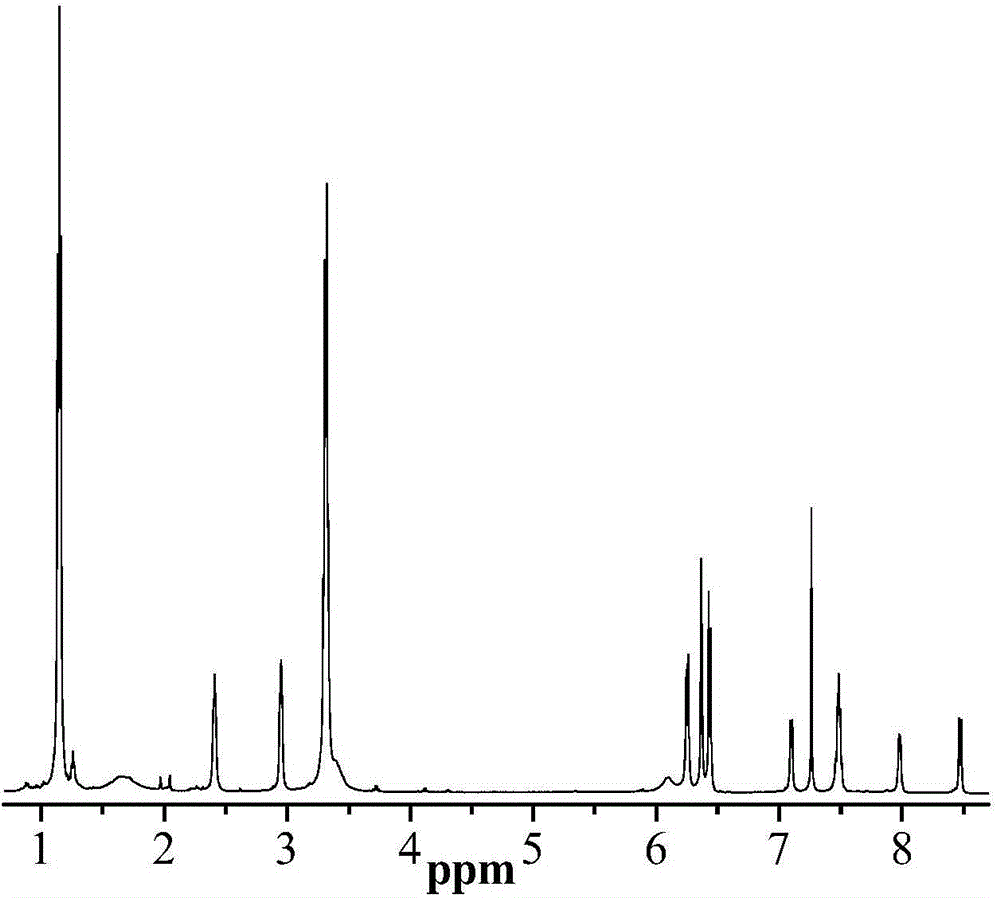
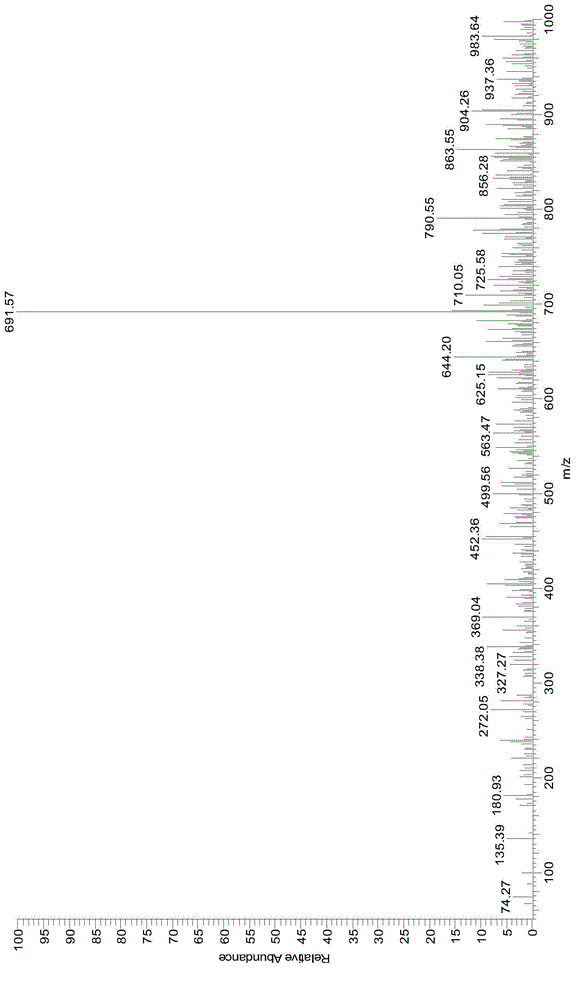
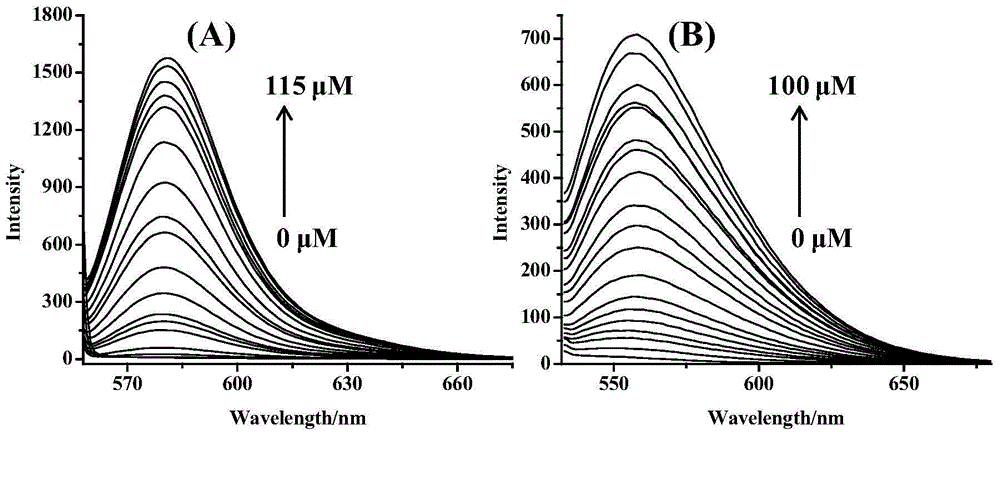
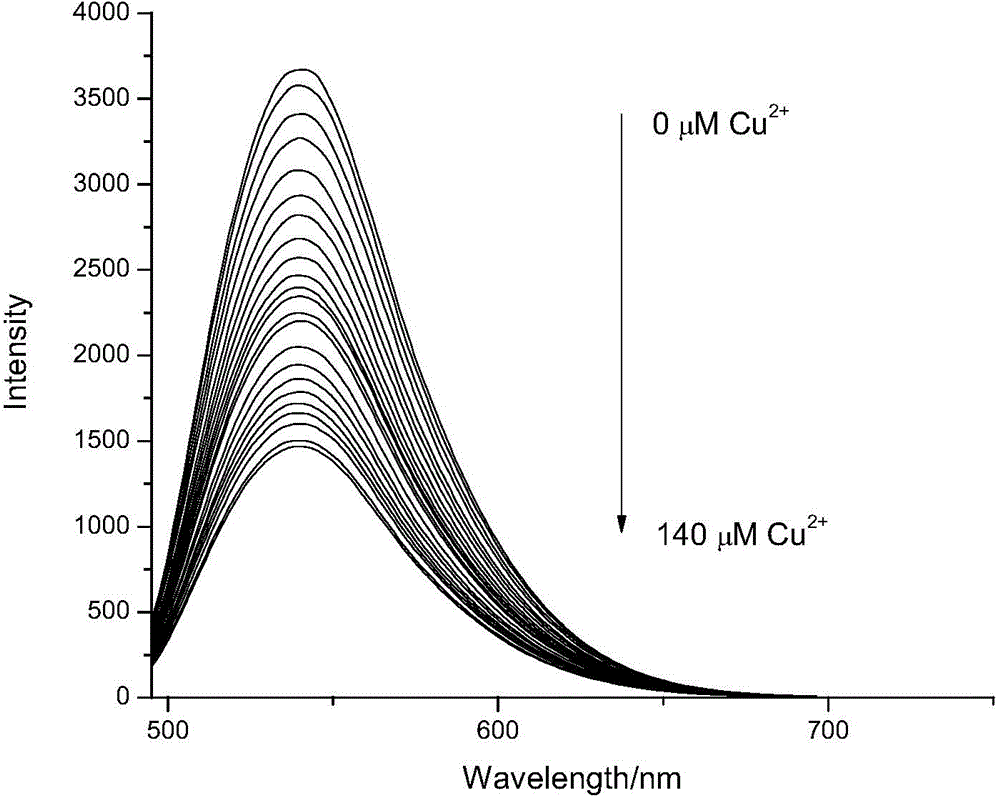
Lysine-modified benzofuroxan compound, synthetic method, application and recovery method of lysine-modified benzofuroxan compound as well as method of detecting concentration of copper ions
InactiveCN104478823AEasy to detectEasy to prepareOrganic chemistryFluorescence/phosphorescenceNitrobenzeneStructural formula
The invention discloses a lysine-modified benzofuroxan compound, a synthetic method, an application and a recovery method of the lysine-modified benzofuroxan compound as well as a method of detecting the concentration of copper ions. The structural formula of the lysine-modified benzofuroxan compound is shown in the specification. The synthetic method of the lysine-modified benzofuroxan compound comprises the following steps: 1) carrying out reaction on methanol, thionyl chloride and lysine to obtain lysine methyl ester hydrochloride; 2) in the presence of an acid binding agent, carrying out reaction on the lysine methyl ester hydrochloride and 4-chlorine-7-nitro-benzofuroxan, and carrying out column chromatography; and 3) dissolving the product obtained in the last step in alkali liquor, extracting for multiple times, regulating a pH value of a water phase, extracting for multiple times, collecting an extractant layer, and carrying out purification. The method of detecting the concentration of the copper ions comprises the following steps: 1) drawing a standard curve; 2) detecting and recording; and 3) carrying out calculation. The recovery method of the lysine-modified benzofuroxan compound comprises the following steps: adding a complexing agent aqueous solution into the compound solution containing the copper ions, uniformly mixing, extracting, collecting an organic phase, and removing a solvent. The lysine-modified benzofuroxan compound is applied to preparing a copper ion fluorescent probe. The synthesized lysine-modified benzofuroxan compound is capable of specifically detecting the copper ions, can be prepared into the probe and can also be recovered.
Owner:SOUTH CHINA NORMAL UNIVERSITY
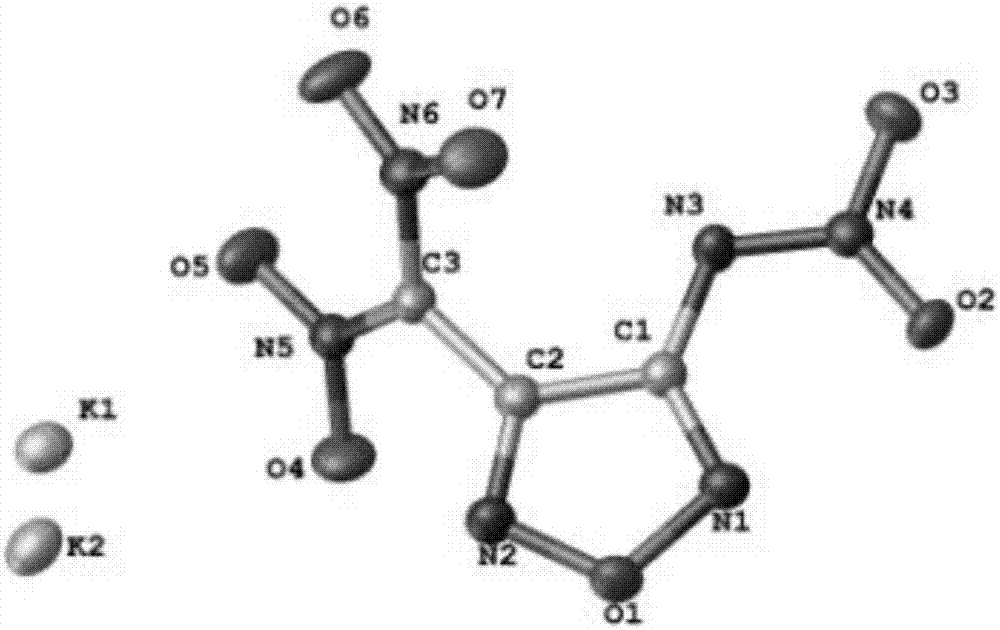
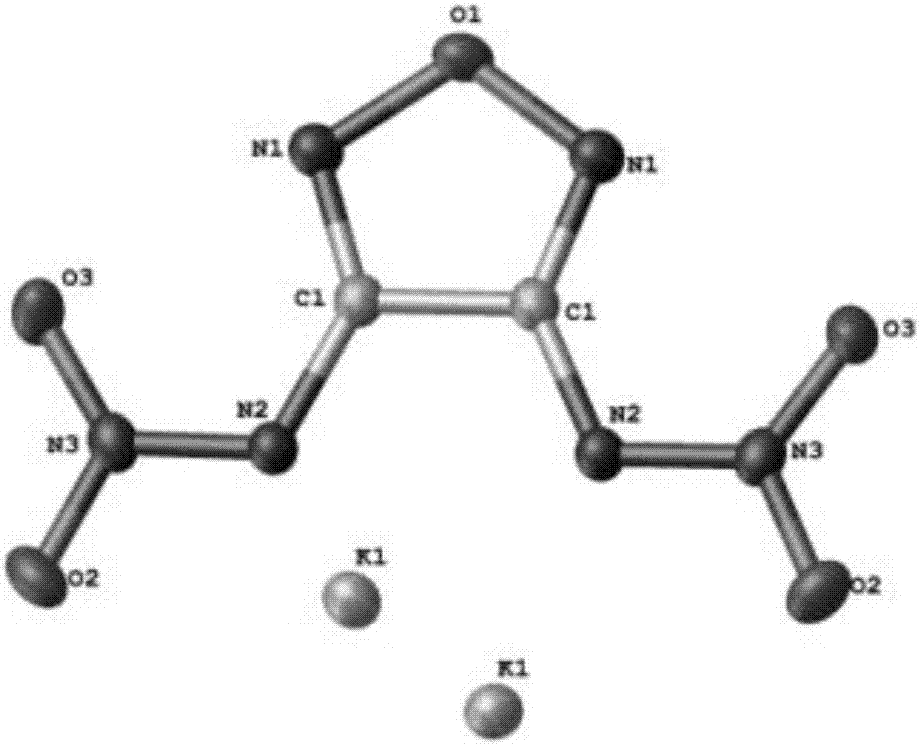
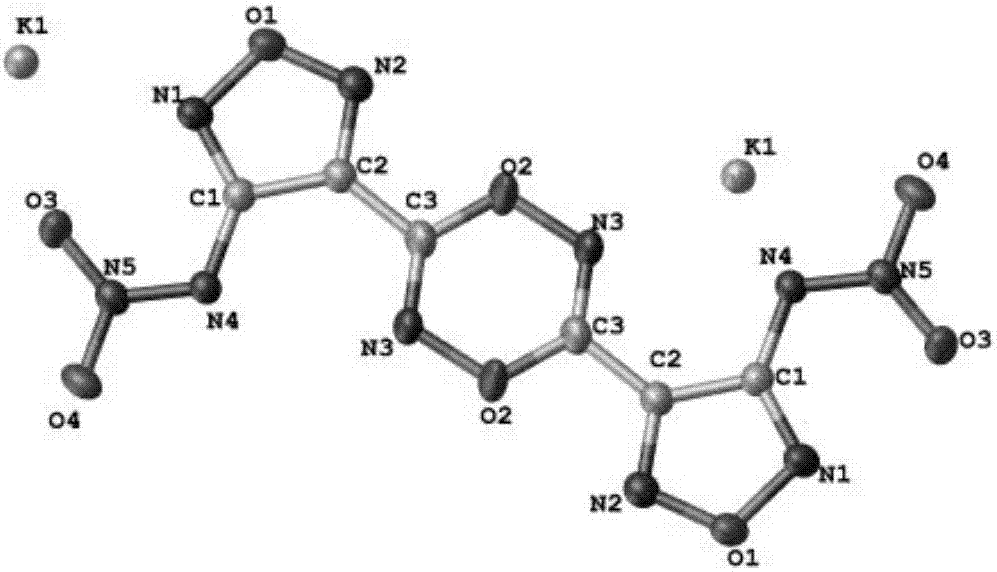
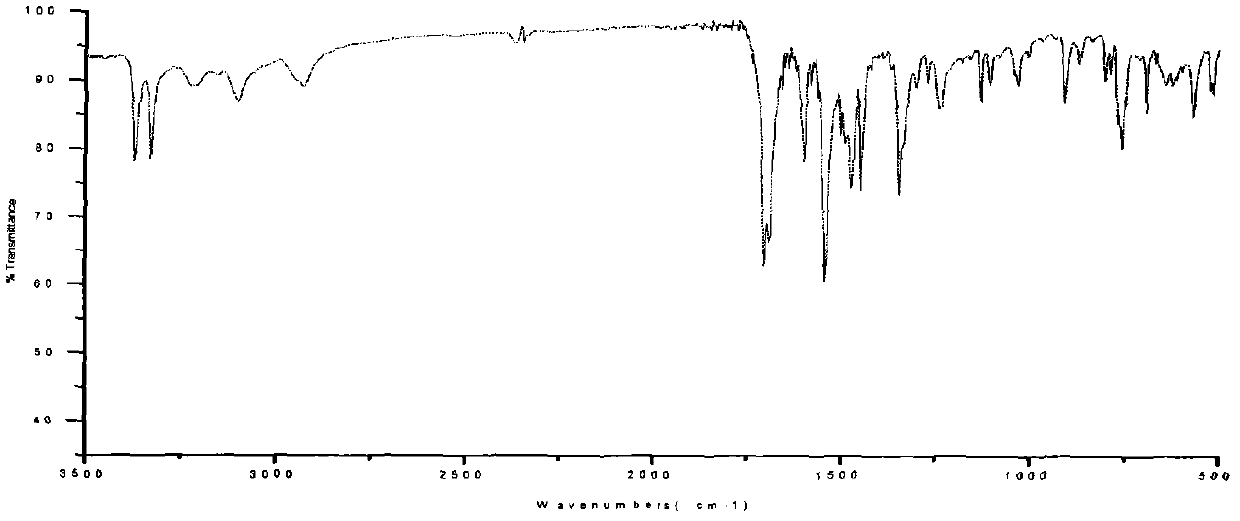
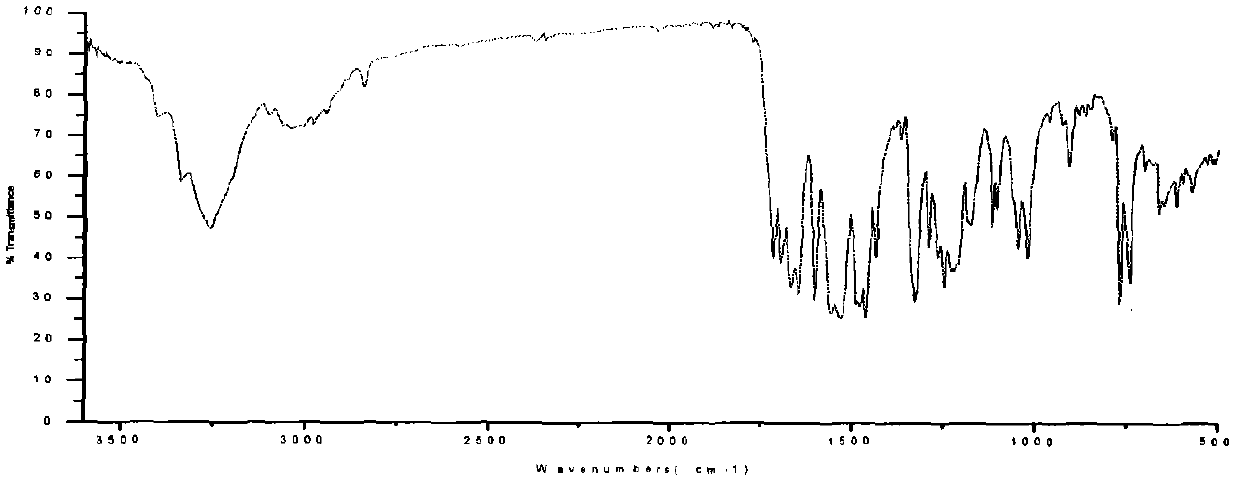
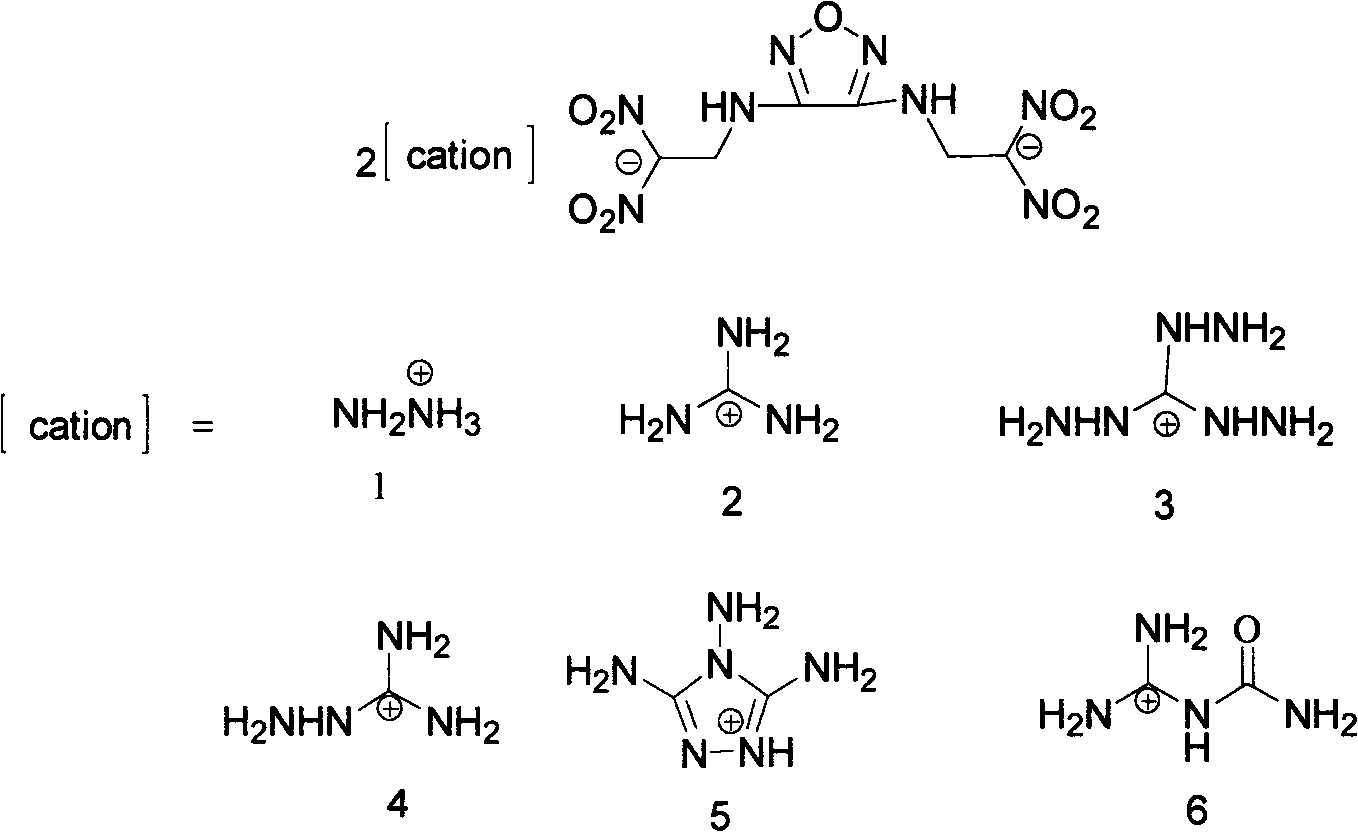
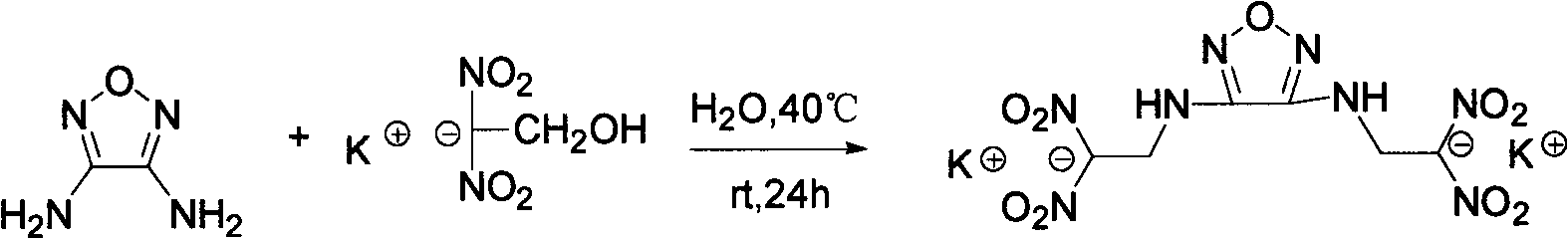
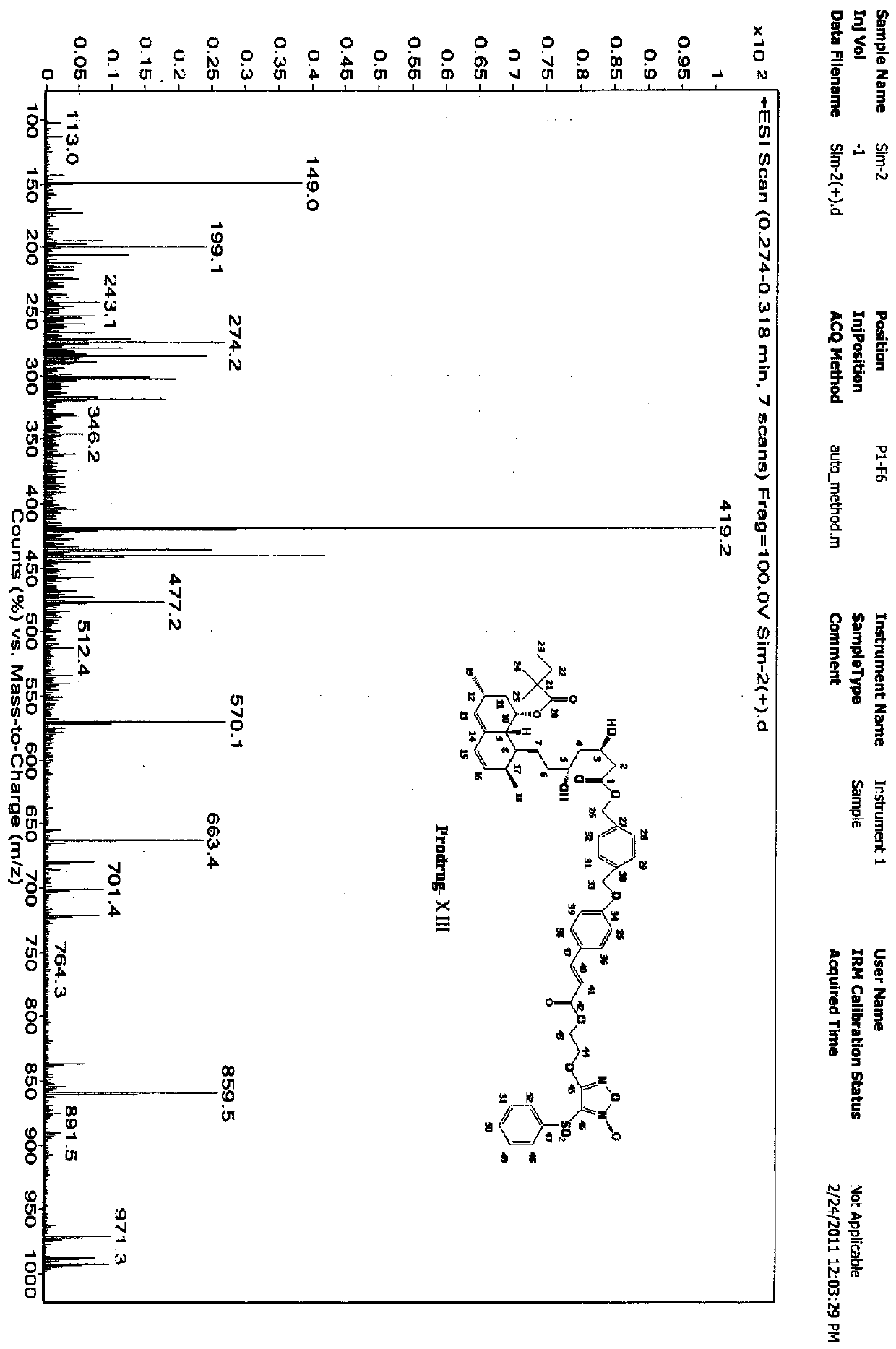
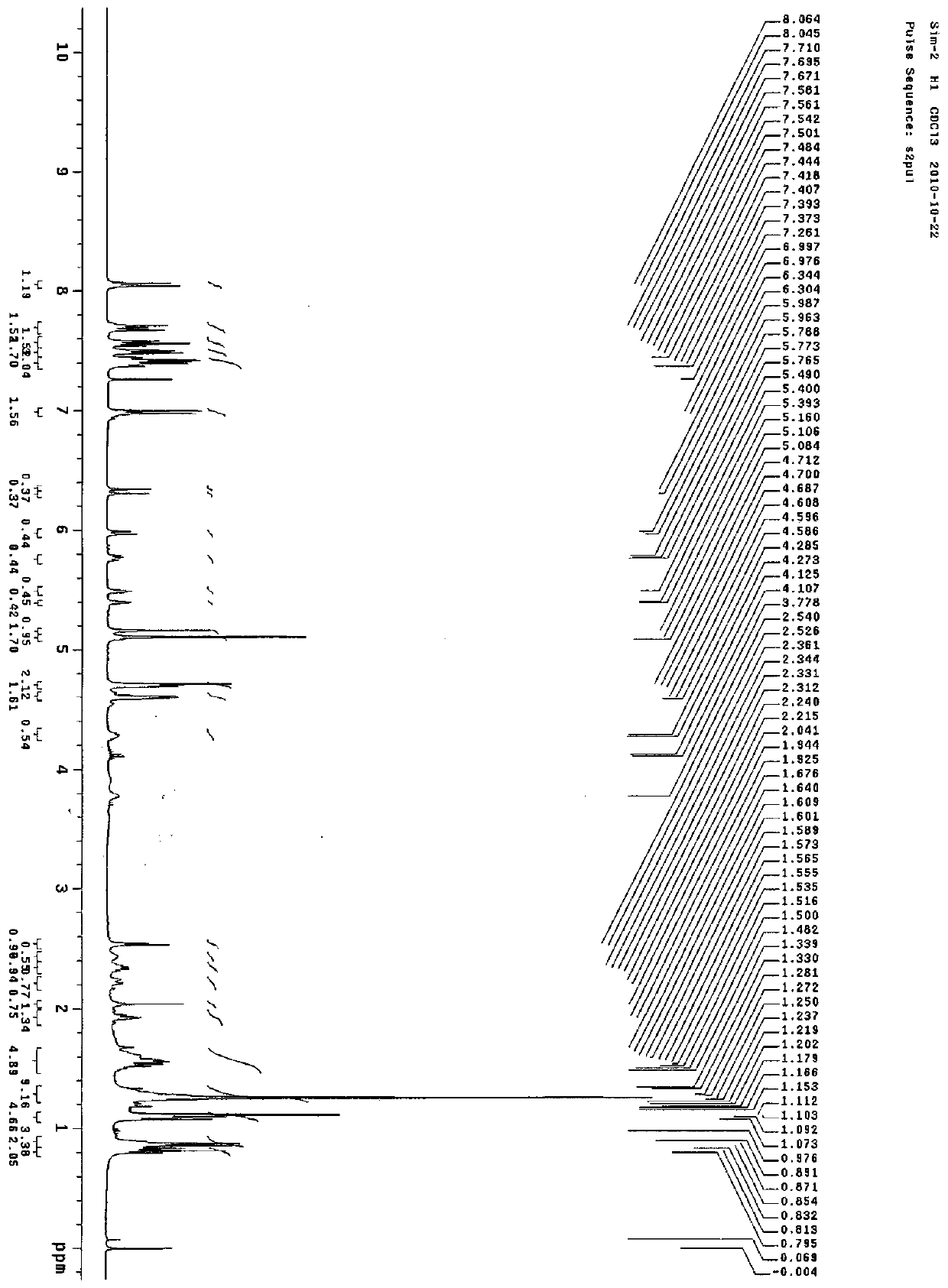
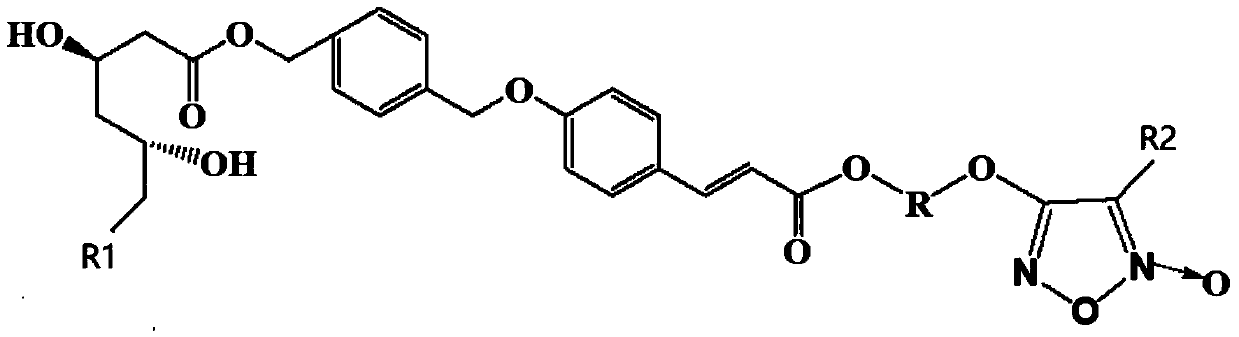
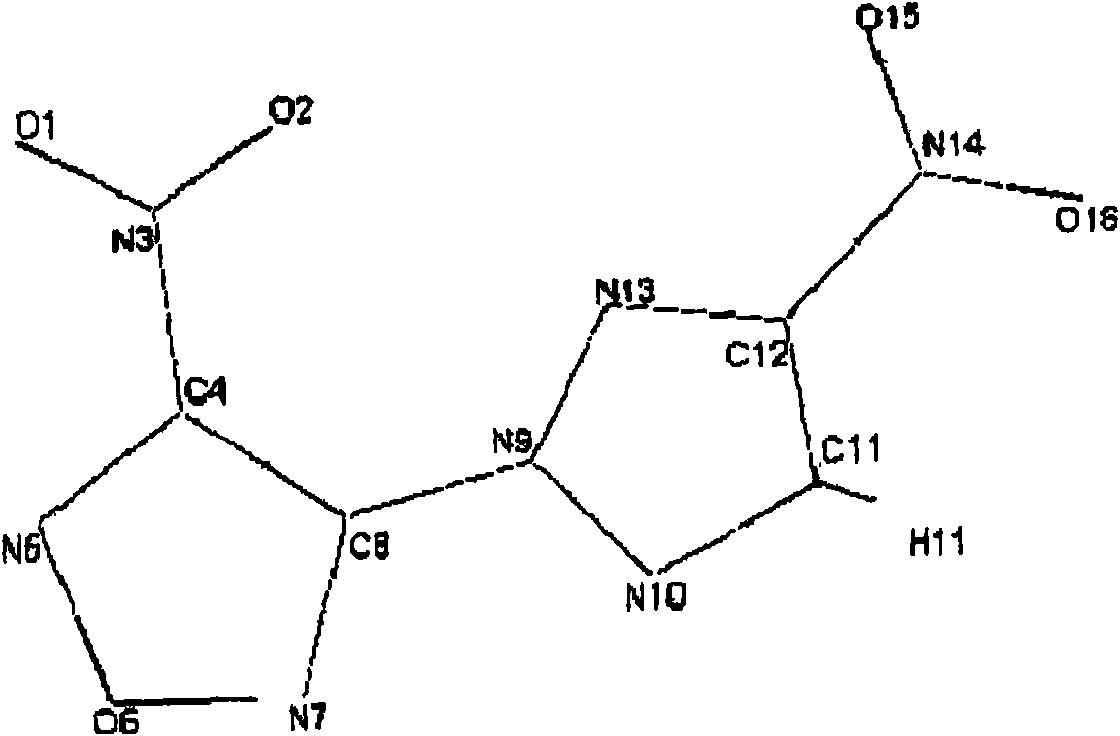
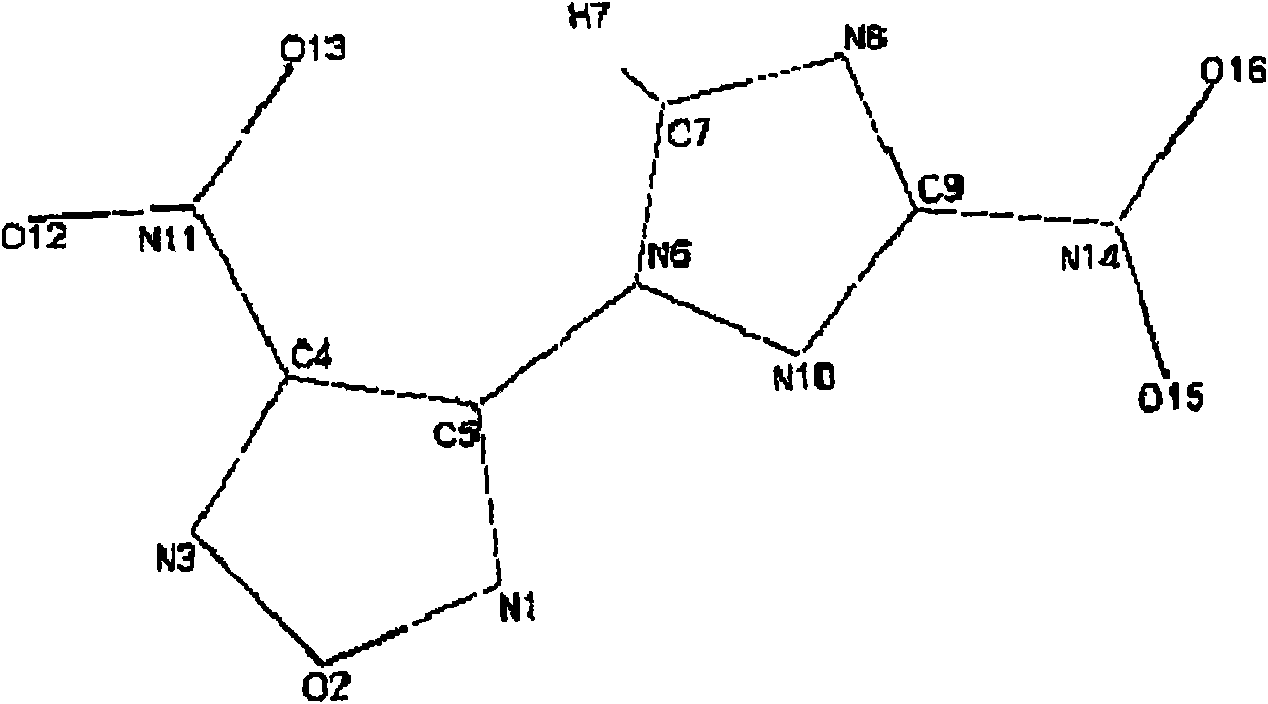
Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof
InactiveCN103483330AImprove thermal stabilityLow impact sensitivityOrganic chemistryOrganic compound preparationFurazanDetonation
The invention discloses energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and a preparation method thereof, and belongs to the technical field of energy containing materials. The preparation method is as follows: mixing 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan barium salt with an equal-molar sulfate of a corresponding cation, filtering precipitates, and evaporating out a filtrate to obtain the target product. The preparation method is simple and easy to industrialize. Nine energy containing ionic salts related in the invention are high in thermostability (Td: 213-289 DEG C), the degree of percussion sensitivity of most compounds is greater than 15J, all the energy containing ionic salts have excellent calculation detonation performance and are potential energy containing materials.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
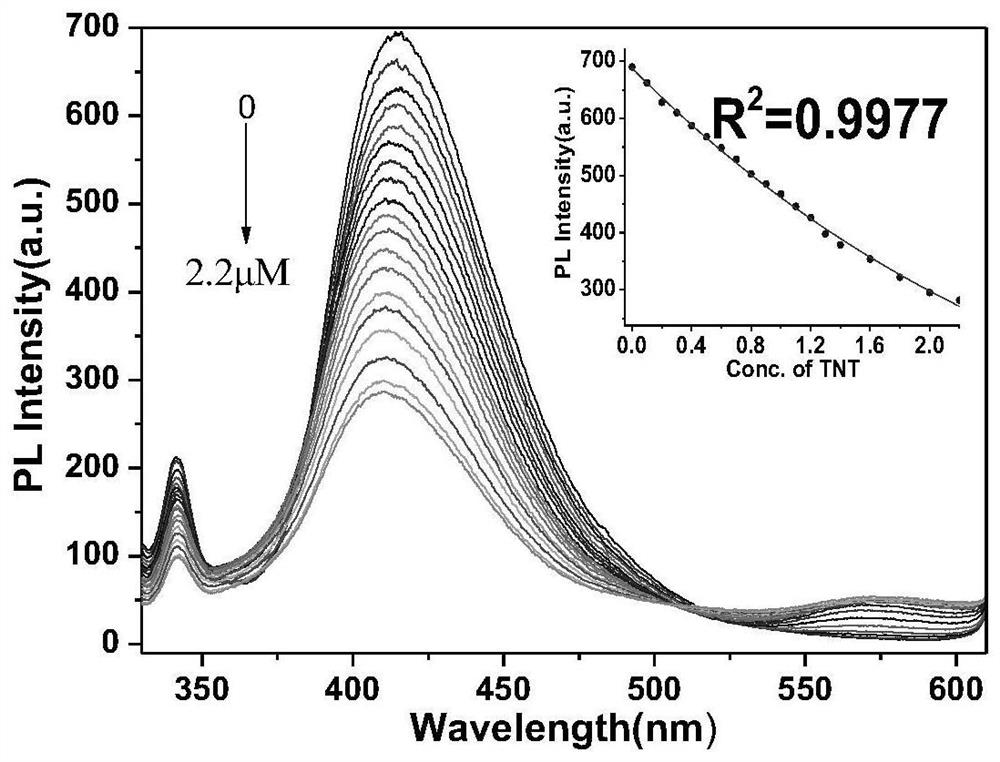
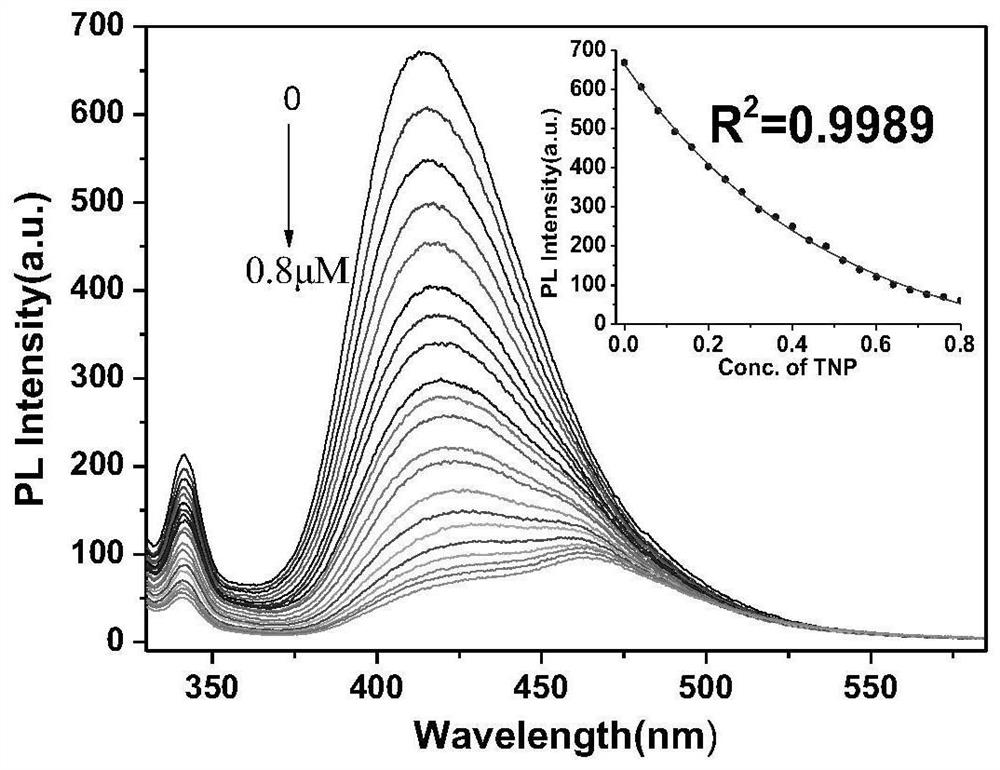
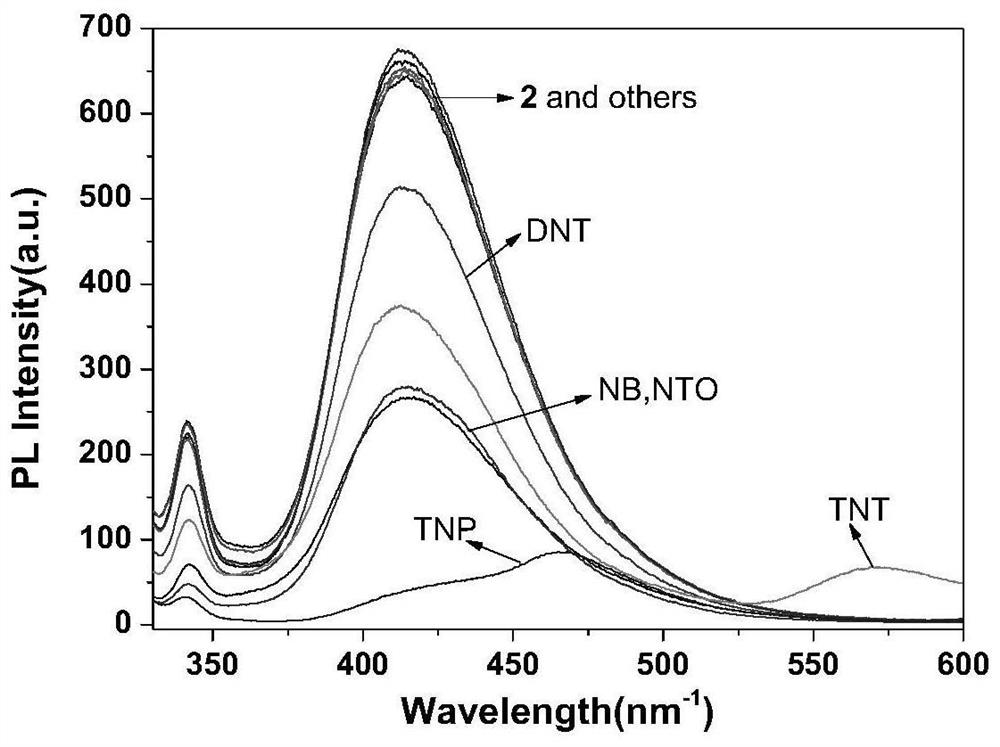
Fluorescent probe capable of simultaneously detecting TNT and TNP and preparation method thereof
ActiveCN111943907AHigh sensitivityStrong specificityOrganic chemistryFluorescence/phosphorescenceSodium bicarbonateFluoProbes
The invention relates to a preparation method of a TNT and TNP detection probe, and belongs to the field of small molecular fluorescent materials. The preparation method comprises the following steps:reacting 3,4-diamino furazan with sodium bicarbonate, and recrystallizing with DMSO / H2O to prepare 3,3'-diamino-4,4'-azofurazan; reacting the 3,3'-diamino-4,4'-azofurazan with 2-hydroxy-1-naphthaldehyde to prepare a difunctional fluorescent molecular probe; and filtering, washing, drying and weighing the product to obtain a dark red solid product, thereby obtaining the final product. The probe realizes simultaneous rapid selective recognition of TNT and TNP on an ultraviolet channel and a fluorescence channel, especially in the fluorescence channel, after TNT and TNP are added, fluorescence intensity of probe molecules is quenched to different degrees, and red shift of different degrees also occurs in an emission peak, so that the probe is very effective for differentially detecting TNT and TNP.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
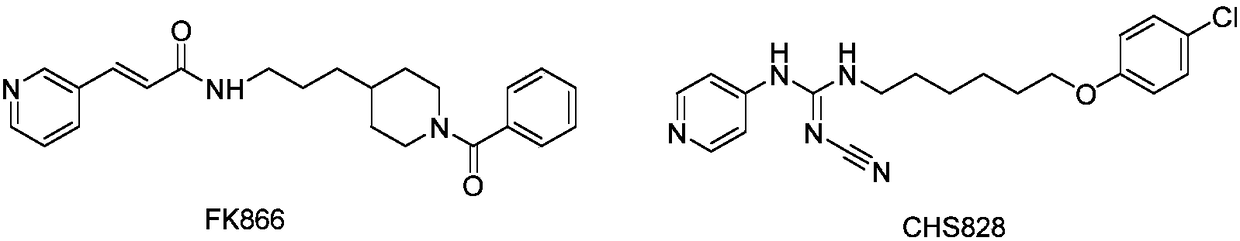
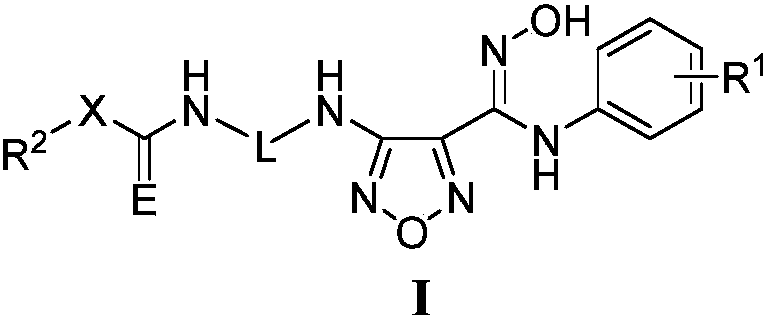
Novel NAMPT and IDO dual inhibitor, and preparation method and medical applications thereof
ActiveCN108530444AGrowth inhibitionPrevent proliferationOrganic chemistryAntineoplastic agentsFurazanDual inhibitor
The invention discloses a novel NAMPT and IDO dual inhibitor, and a preparation method and medical applications thereof, and more specifically relates to a furazan compound, or pharmaceutically acceptable salts, solvates, prodrugs, esters, racemic compounds, and isomers of the furazan compound, and pharmaceutical compositions containing the furazan compound, and applications of the furazan compound or the pharmaceutical compositions in the field of anti-tumor drug preparation. According to the preparation method, reasonable combination of active segments of an IDO inhibitor and a NAMPT inhibitor is adopted so as to obtain the furazan kind IDO / NAMPT double target inhibitor. On one hand, the furazan kind IDO / NAMPT double target inhibitor is capable of realizing dual inhibition of NAD<+> biosynthesis, and possesses higher anti-tumor inhibition activity, and one the other hand, inhibition of IDO activity is capable of promoting T cell propagation effectively, so that the impurity of bodies on tumor cell attacks is improved. The furazan kind IDO / NAMPT double target inhibitor or the pharmaceutical compositions of the furazan kind IDO / NAMPT double target inhibitor are promising in application prospect, and are promising to be developed into anti-tumor drugs.
Owner:YAOKANG ZHONGTUO (JIANGSU) PHARMA TECH CO LTD
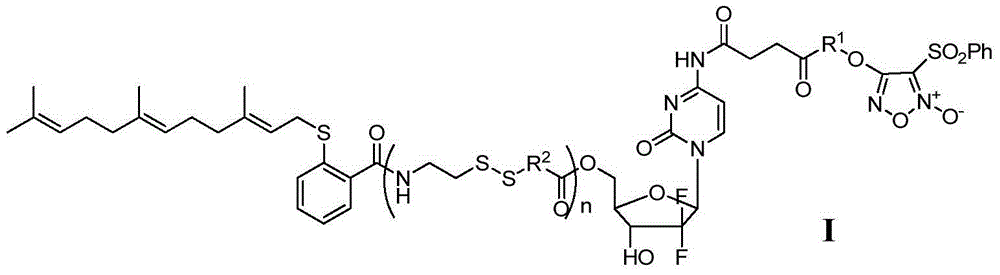
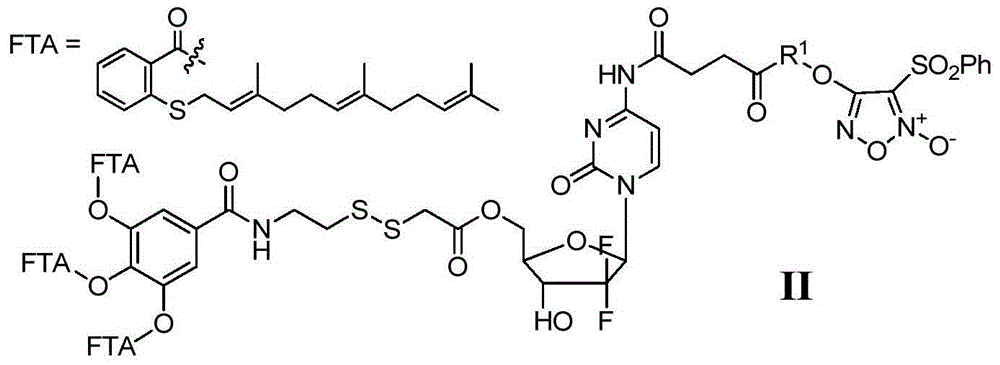
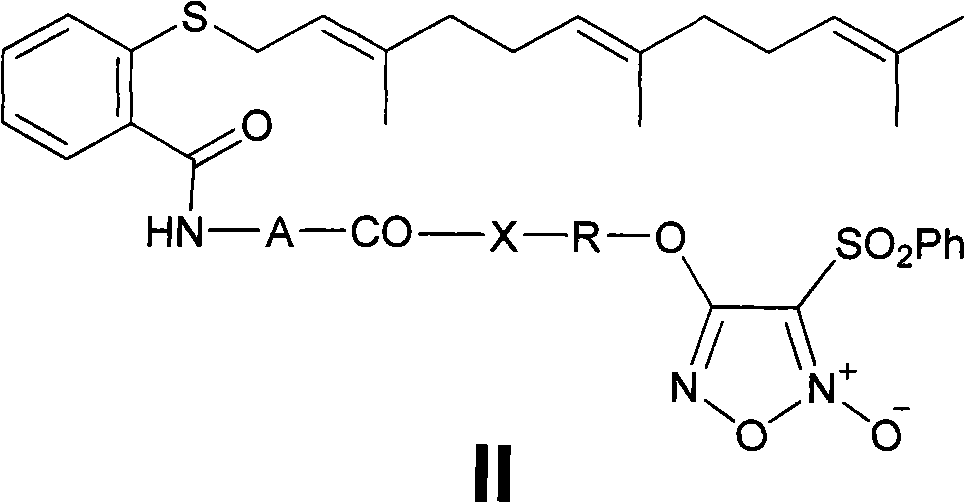
Gemcitabine/FTA/furazan conjugate in NO-donor type, preparation method and application
The invention discloses a gemcitabine / FTA / furazan conjugate in a NO-donor type, a preparation method and an application. A product in the invention is the novel gemcitabine / FTA / furazan conjugate in the NO-donor type or a pharmaceutically-acceptable salt thereof represented as the general formula I and the general formula II. The product has excellent functions of treating common tumor and drug-resistant tumor proliferation, can achieve a synergistic treatment effect of a plurality of drug fragments and is easy to prepare.
Owner:苏州康纯医药科技有限公司
Nitramine furazan energetic potassium salt, and preparation method and application thereof
ActiveCN106928161AOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsPotassiumNitration
The invention provides a nitramine furazan energetic potassium salt, and a preparation method and the application thereof. The nitramine furazan energetic potassium salt has the structure as shown in the description and can be prepared by a one-pot method of nitration of an aminofurazan nitration precursor and treatment of potassium salt. The nitramine furazan energetic potassium salt has the characteristics of being high in oxygen balance, high in energy and high in content of potassium, can be used as a green primary explosive, and can be applied to flash suppression in a solid propellant.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Azo-furazan compound and preparing method thereof
The invention discloses an azo-furazan compound and a preparing method thereof. The preparing method includes the specific steps that malononitrile serves as a raw material and is reacted with an oxidizing agent to obtain an amino-oximido furazan intermediate; the amino-oximido furazan intermediate is reacted with different cyclization reagents (triethyl orthoformate, bromized nitrile, acetic anhydride, trifluoroacetic anhydride and the like) to obtain an amidogen-substituted furazan intermediate, then the furazan intermediate is reacted with potassium permanganate and hydrochloric acid of 10%-20%, and the azo-furazan compound is separated and precipitated. The preparing method is simple; compared with the prior art, azo-furazan compounds with different substituent groups can be prepared at a time in a high throughput mode, operation is safe, and cost is low.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Method for synthesizing plasticizer 3-nitro furazan-4-methyl ether
InactiveCN102702131AOrganic chemistryPressure gas generationHydroxylamine HydrochlorideEthyl fumarate
The invention discloses a method for synthesizing plasticizer 3-nitro furazan-4-methyl ether. The method comprises the following steps: adding hydroxylamine under the alkaline condition by taking ethyl cyanoacetate as a starting material to form a ring, so as to obtain 3-amino furazan-4-carboxylic acid; then, reducing carboxyl group on the furazan ring to an alcoholic extract hydroxyl group by using a sodium borohydride / zinc chloride system, so as to obtain 3-amino furazan-4-methanol; then, oxidizing an amino group on the furazan ring to a nitro group by using a hydrogen peroxide / concentrated sulphuric acid system, so as to obtain 3-nitro furazan-4-methanol; in the presence of pyridine, carrying out chloride substituent on the alcoholic extract hydroxyl group by using thionyl chloride, so as to obtain 3-nitro-4-chloromethyl methyl ether furazan; and finally, reacting 3-nitro-4-chloromethyl methyl ether furazan with sodium methoxide in acetonitrile to remove NaCl so as to obtain the target compound. Compared with the traditional nitrate plasticizer, the plasticizer synthesized by using the method has higher positive enthalpies of formation and thermal stability, lower sensitivity and melting point, and has potential application prospect in the field of solid propellants.
Owner:太仓市新星轻工助剂厂
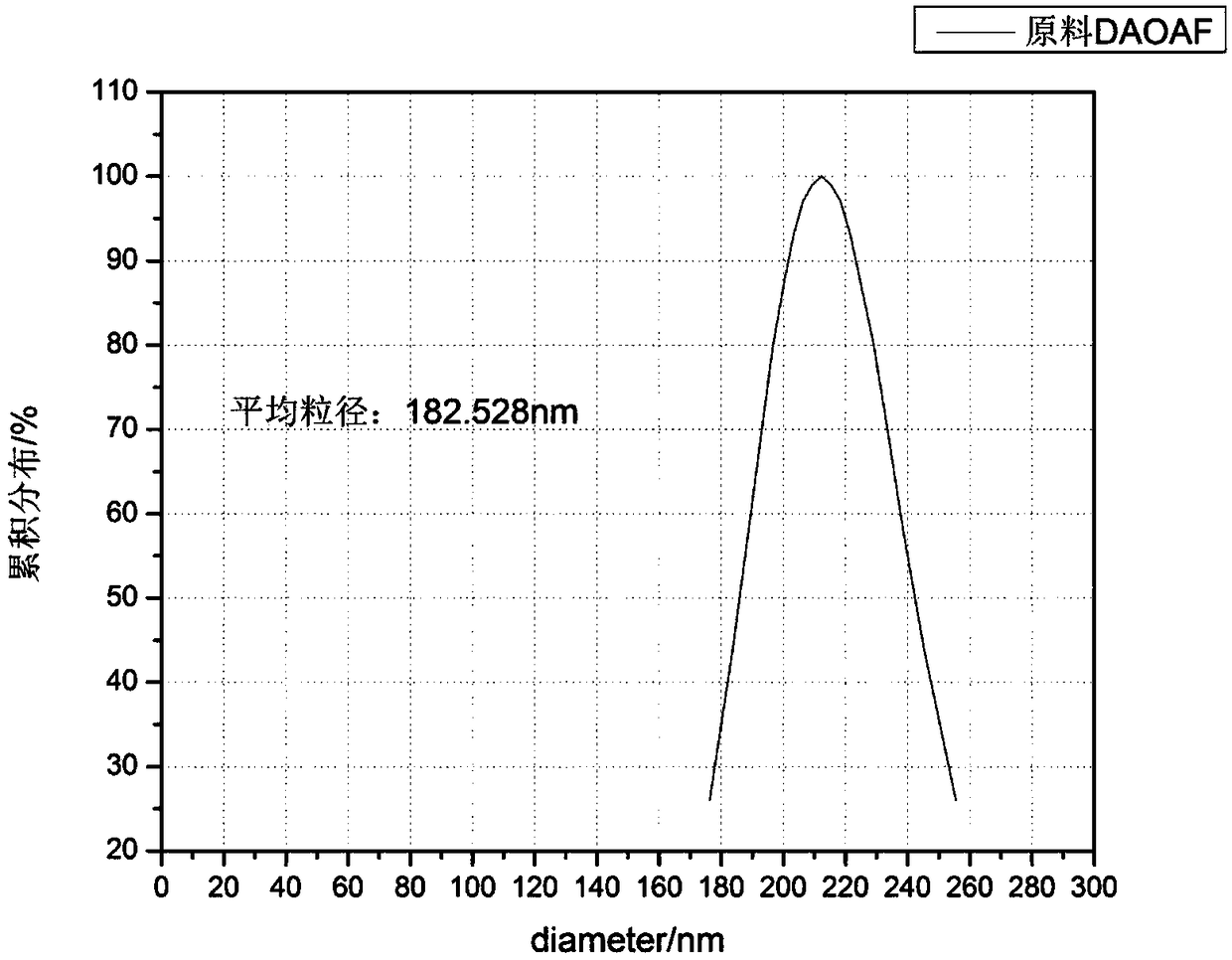
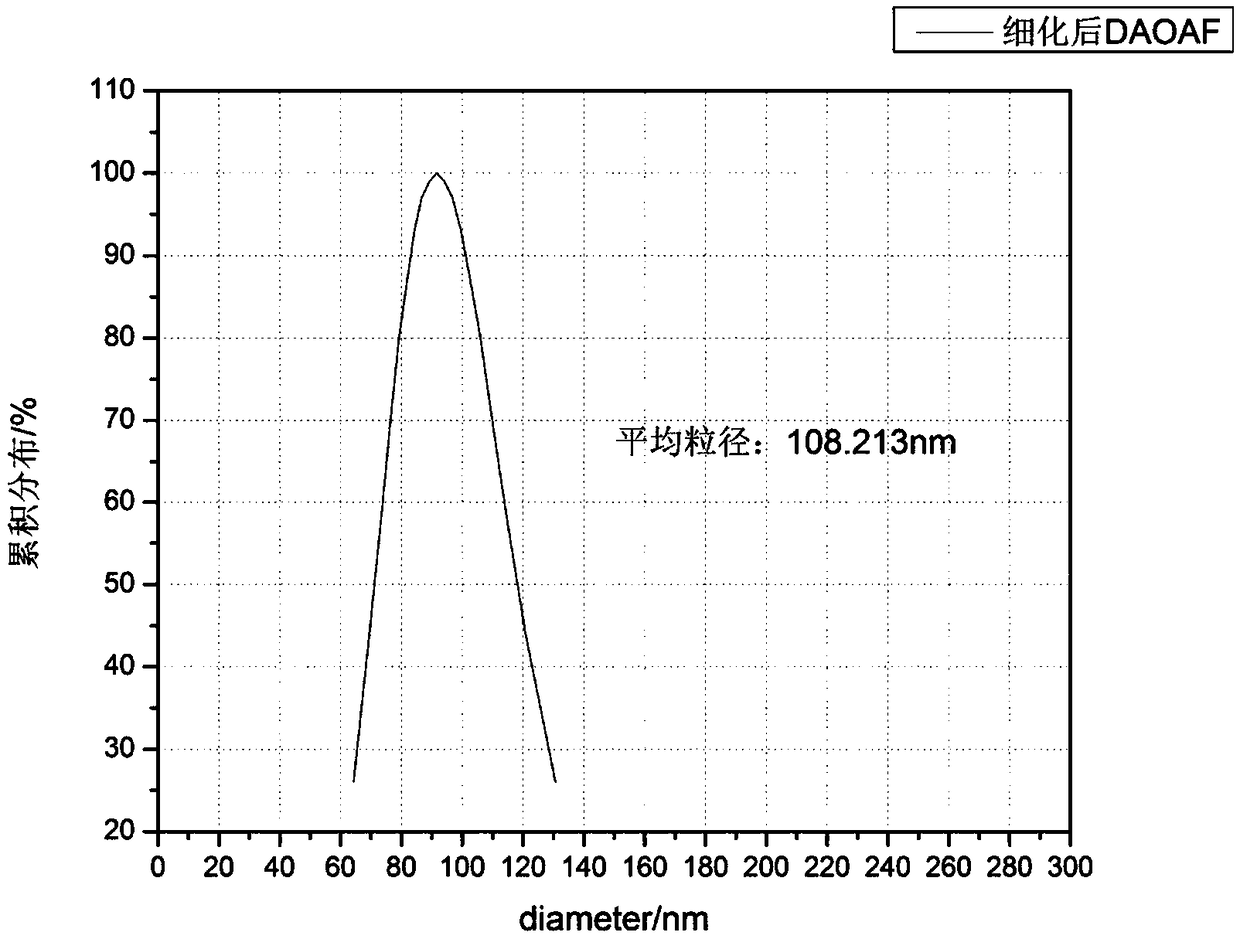
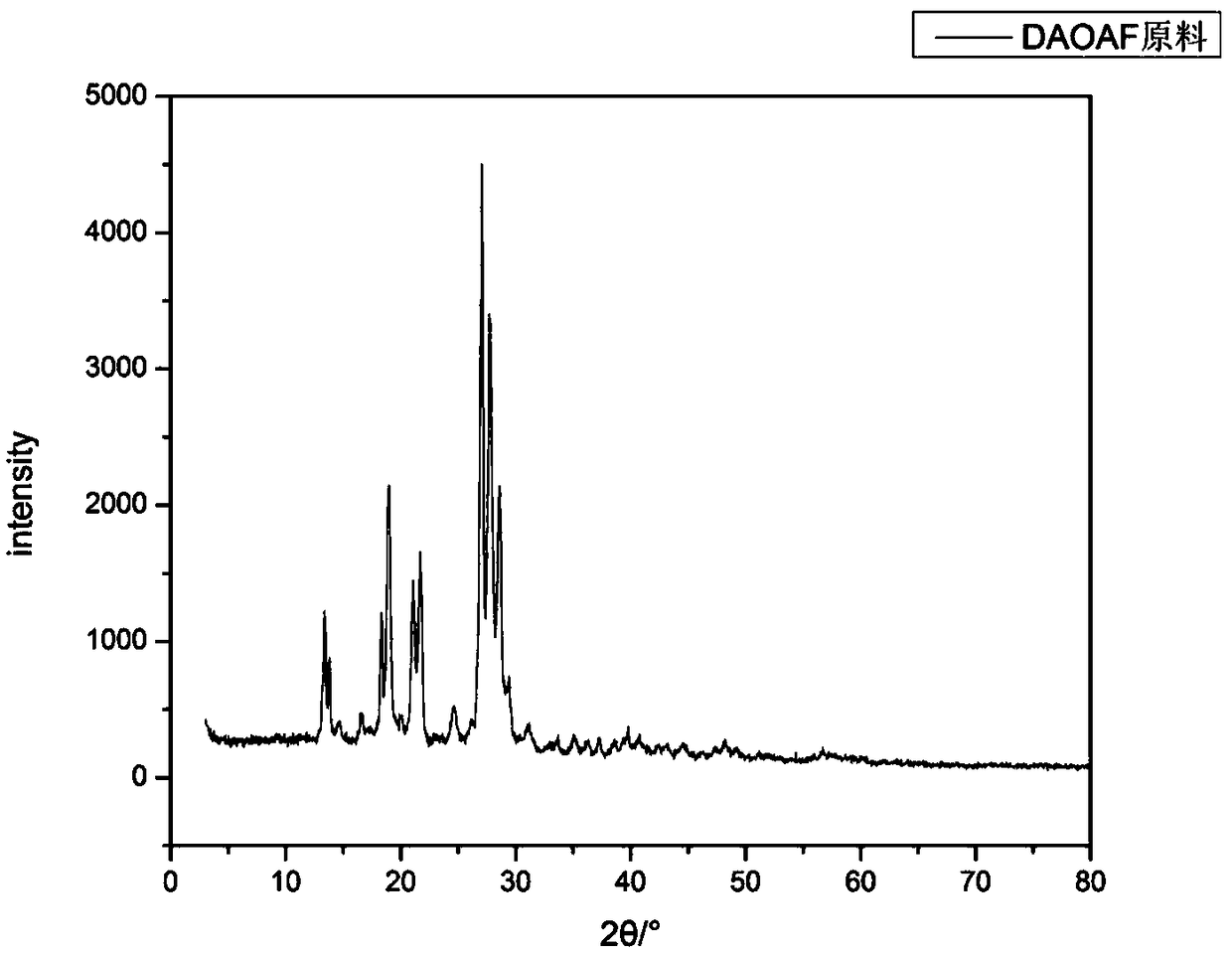
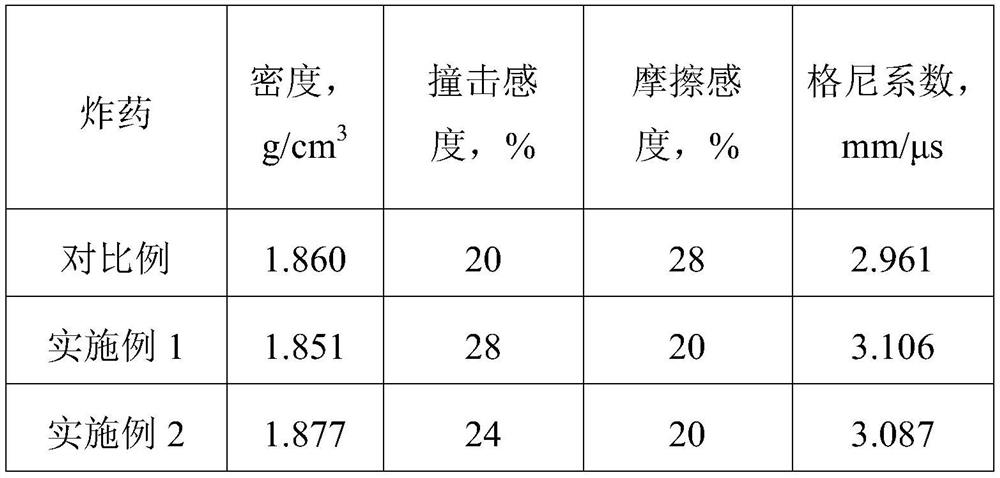
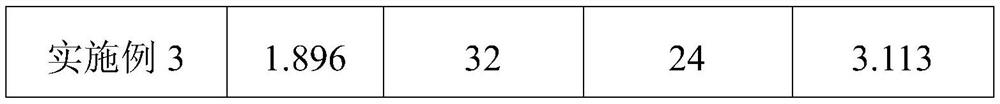
Refining method of DAOAF (3,3'-diamino-4,4'-azoxy furazan) explosive crystal
The invention relates to the field of energy-containing materials, in particular to a refining method of a DAOAF (3,3'-diamino-4,4'-azoxy furazan) explosive crystal. The refining method comprises thefollowing steps of (1) dissolving the raw materials of DAOAF and DMSO (dimethyl sulfoxide) by ultrasonic waves to form a DAOAF solution, and pouring the DAOAF solution into an atomizing spray head; (2) opening nitrogen gas to pressurize, enabling the DAOAF solution to form small mist droplets by the atomizing spray head, enabling the small mist droplets to be in contact with a non-solvent, stirring by the ultrasonic waves, and quickly crystallizing and separating DAOAF particles; (3) stirring the DAOAF particles by ultrasonic waves, filtering, and drying under the vacuum condition; (4) enabling a laser granularity meter and a SEM (scanning electron microscope) to perform granularity and morphology characterization on the re-crystallized DAOAF particles. The refining method has the advantages that the solvent can be quickly removed by a spray anti-solvent method, the materials are further crushed by stirring and ultrasonic waves, and the DAOAF crystal particles can be easily refined athigh efficiency; the refined DAOAF has better development prospect in the fields of slapper detonator primers, high-energy mixed explosives, and high-energy solid propellants.
Owner:SOUTHWEAT UNIV OF SCI & TECH +1
Metal accelerated explosive and preparation method thereof
The invention discloses a metal accelerated explosive and a preparation method thereof, and aims to solve the problem of low acceleration capability of the existing metal acceleration type explosive.The metal accelerated explosive comprises the following components in percentage by mass: 75%-83% of 3, 4-dinitrofurazan-based furazan oxide (D5030 [mu]m to 40 [mu]m), 15%-20% of nano aluminum powder(D50100nm to 200nm), 1%-4% of ethylene-vinyl acetate copolymer (EVA, model 28 / 150) and 1%-4% of paraffin (68 #). The preparation method comprises the following steps: preparing composite particles ofnano aluminum powder and 3, 4-dinitrofurazan-based furazan oxide (DNTF), and carrying out coating granulation by using EVA and paraffin to complete the preparation of the metal accelerated explosive molding powder. According to the explosive, on the premise that safety is guaranteed, high metal driving capacity can be achieved. The charging device is suitable for charging of armor-breaking warheads and explosion-killing warheads.
Owner:XIAN MODERN CHEM RES INST
Quinoxaline-N1,N4-dioxide derivative capable of inhibiting activity of DNA topoisomerase, preparation method and application of quinoxaline-N1,N4-dioxide derivative
PendingCN110551072AHas inhibitory activityStrong inhibitory activityAntibacterial agentsOrganic chemistryQuinoxalineStaphylococcus aureus
The invention belongs to the technical field of biochemistry, and particularly relates to a quinoxaline-N1,N4-dioxide derivative capable of inhibiting the activity of DNA topoisomerase, a preparationmethod and application of the quinoxaline-N1,N4-dioxide derivative. 4,5,-difluoro-2-nitroaniline is used as a raw material for synthesis of the quinoxaline-N1,N4-dioxide derivative, the quinoxaline-N1,N4-dioxide derivative reacts with sodium hypochlorite under catalysis of a basic catalyst, namely sodium hydroxide, and 5,6-difluoro-N-benzofuroxan is obtained; and then the quinoxaline-N1,N4-dioxidederivative reacts with different substrates in Beirut reaction and substitution reaction, and a series of the quinoxaline-N1,N4-dioxide derivative is obtained. According to the quinoxaline-N1,N4-dioxide derivative, the preparation method and application of the quinoxaline-N1,N4-dioxide derivative, quinoxoline-N1,N4-dioxide has good bacteriostatic activity to previously reported gram-negative bacteria, and also had good bacteriostatic activity to actinobacilluspleuropneumoniae and gram-positive bacteria such as staphylococcus aureus and streptococcus pneumoniae.
Owner:HUAZHONG AGRI UNIV
Dioxoquinoxaline formamidourea with plant growth regulating activity, preparation method and application thereof
The invention specifically relates to a compound with a plant growth regulating activity, a preparation and an application thereof. The compound contains quinoxaline formamidourea as shown in the general formula 1, in which R is an alkyl, phenyl or substituted phenyl group. The substituted phenyl group can be a substituted or multi-substituted phenyl group, wherein R' on the multi-substituted phenyl group can be the same and can also be different; the position of R' group on the phenyl group is any one or more positions selected from 2, 3, 4, 5 and 6- positions, and the substituent group is methyl, nitryl, alkoxide, halogen atom or fluoroform. The preparation method comprises the following steps of: performing two-step reaction on benzofurazan used as a raw material to obtain a quinoxaline formylhydrazine intermediate; performing a reaction between amine and solid phosgene to synthesize a rotaxane formation intermediate, and performing a reaction between the quinoxaline formylhydrazine intermediate and the rotaxane formation intermediate to obtain the compound as shown in the general formula 1. The invention also discloses a preparation of the intermediates. The compound as shown in the general formula 1 has an obvious effect of regulating the growth of monocotyledons and dicotyledons, and can be used as a growth regulator for monocotyledon and dicotyledon weeds.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
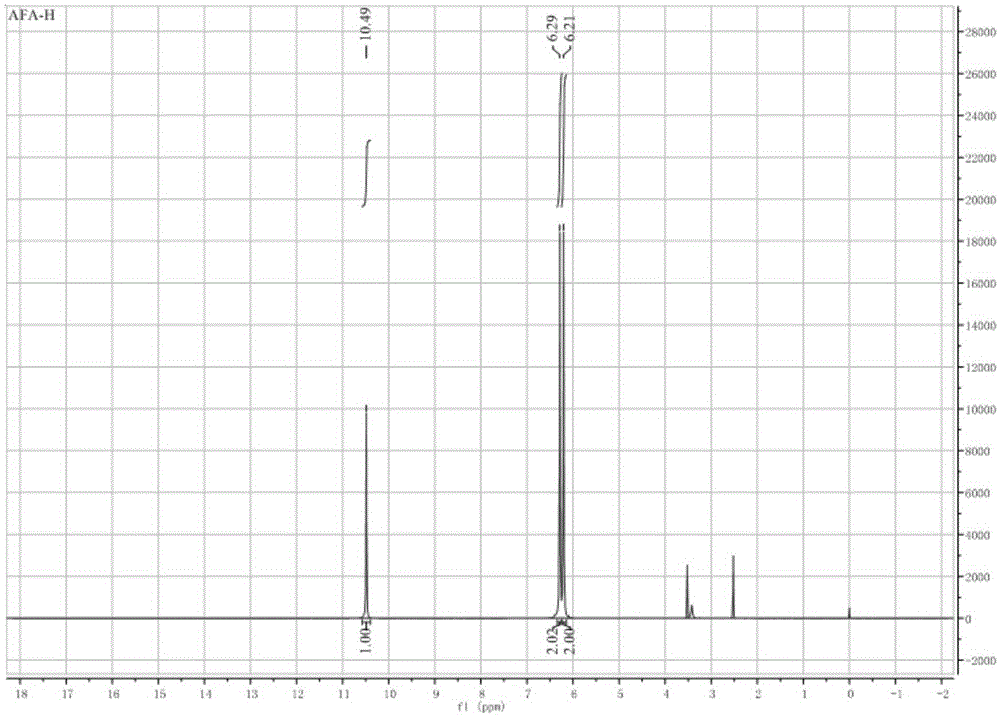
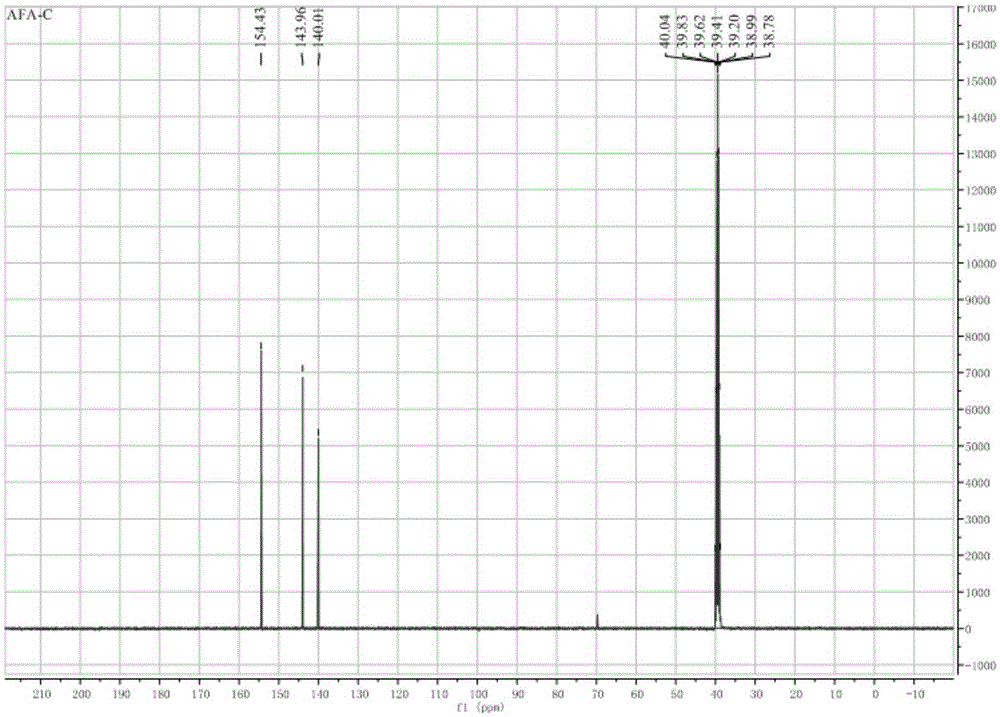
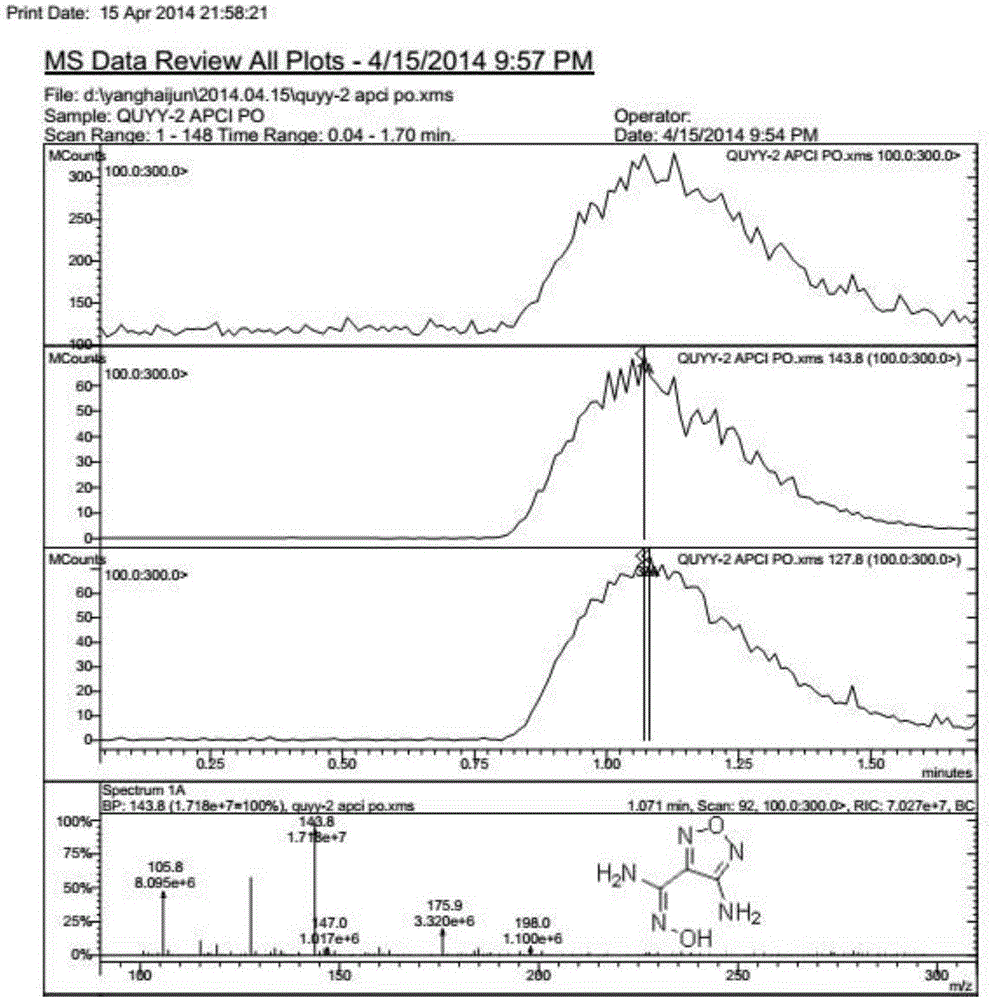
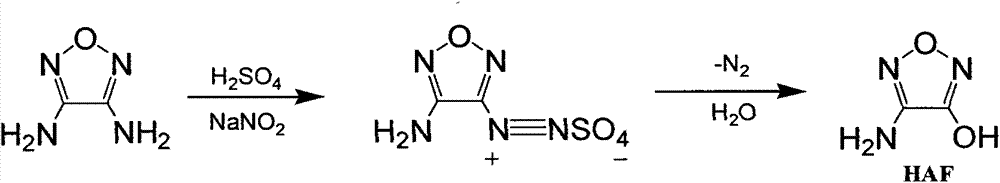
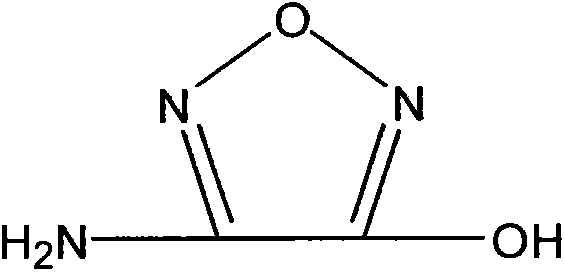
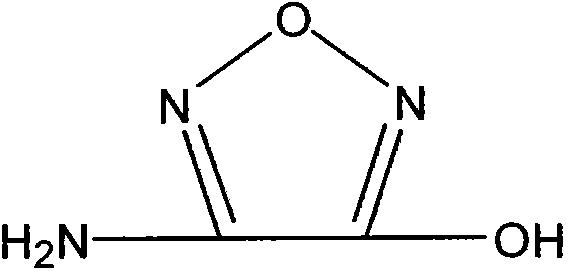
Synthetic method of 3-amino-4-hydroxyfurazan
The invention discloses a synthetic method of 3-amino-4-hydroxyfurazan. The structure of 3-amino-4-hydroxyfurazan is represented by a formula shown in the specification. The method which treats 3,4-diaminofurazan as a raw material comprises the following steps: adding 3,4-diaminofurazan and 20-30% (mass) sulfuric acid into a reaction bottle under stirring, adding an aqueous solution of sodium nitrite into the reaction bottle at -5-5DEG C in a dropwise manner under a condition that the addition time is controlled in a range of 1.5-2.5h, reacting at -5-5DEG C for 2-3h after the addition, naturally heating to 20-25DEG C under stirring, carrying out water-bath heating to 40-60DEG C, reacting for 10-20min, cooling to 0-5DEG C, stirring for 20-40min, filtering, washing the obtained filter cake, and drying to obtain 3-amino-4-hydroxyfurazan. The method is mainly used for preparing 3-amino-4-hydroxyfurazan.
Owner:XIAN MODERN CHEM RES INST
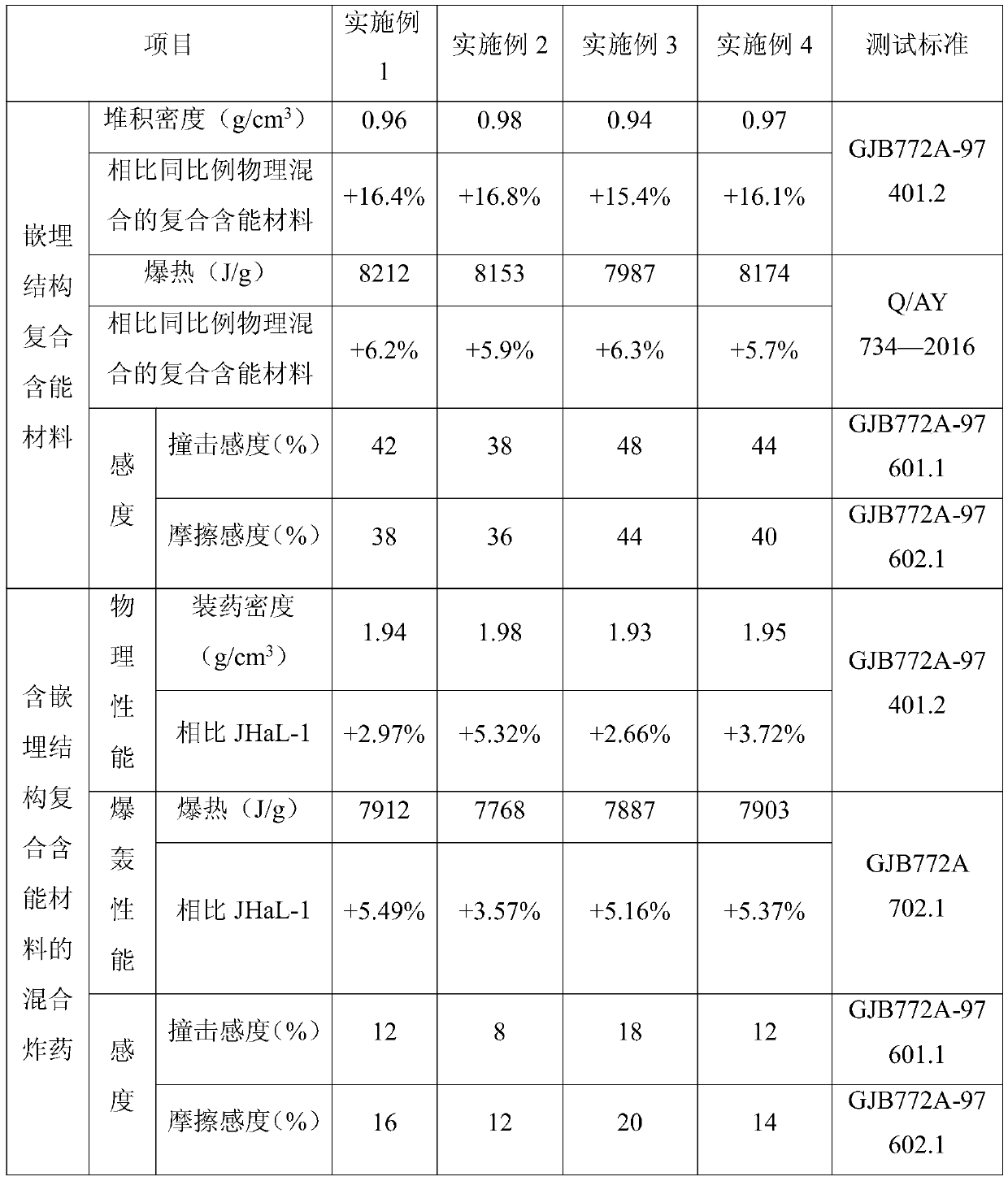
Composite energetic material with embedded structure and preparation method thereof
ActiveCN111470924AShorten the diffusion distanceHigh bulk densityExplosive ingredient compoundingFurazanExplosive Agents
The invention discloses a composite energetic material with an embedded structure and a preparation method of the composite energetic material. A 3,4-dinitrofurazan-based furazan oxide / aluminum powdercomposite energetic material with an embedded structure is prepared by adopting a solvent-non-solvent method; 3,4-dinitrofurazan-based furazan oxide is dissolved into ethyl acetate, the solution is dropwise added into n-hexane turbid liquid of aluminum powder, and the dropwise adding speed, the stirring speed and the preparation time are controlled to obtain the embedded composite energetic material. The method provided by the invention can be used for effectively reducing the diffusion distance between 3,4-dinitrofurazanyl furazan oxide and aluminum powder, the crystal morphology and the surface state of 3,4-dinitrofurazanyl furazan oxide are optimized, the prepared composite energetic material has the advantages of being high in stacking density, low in sensitivity and high in detonation heat, and the detonation heat of the 3,4-dinitrofurazanyl furazan oxide pressed aluminum-containing explosive can be effectively improved. The composite energetic material having the embedded structure is prepared from the following components in percentage by mass: 60 to 75 percent of the 3,4-dinitrofurazanyl furazan oxide and 25-40% of the aluminum powder.
Owner:XIAN MODERN CHEM RES INST
Diazo(N-dinitryl ethyl) furazan energy-containing ionic salts and preparation method thereof
InactiveCN103121977AHigh densityImprove detonation performanceOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsDetonationOrganic base
The invention relates to diazo(N-dinitryl ethyl) furazan energy-containing ionic salts and a preparation method thereof, and belongs to the technical field of energy-containing materials. The synthetic method comprises the following steps of: performing acid-base neutralization reaction of diazo(N-dinitryl ethyl) furazan and 2 times of organic base in mole to obtain corresponding energy-containing ionic salts or performing reaction of diazo(N-dinitryl ethyl) furazan sylvite with 2 times of hydrochloride in mole of corresponding cations; and filtering the precipitate to obtain the target product. The synthetic method provided by the invention is simple and easy to industrialize. 6 energy-containing ionic salts are better in thermal stability, the calculated detonation performance of the energy-containing ionic salts is far superior to that of TNT (Trinitrotoluene), and the detonation performance of part of compounds is approximate to that of RDX (Cyclotrimethylene Trinitramine). The diazo(N-dinitryl ethyl) furazan energy-containing ionic salts can be used as substituents of a nitroform explosive and a furazan explosive for improving oxygen balance in a mixed explosive and used as an oxygen supply component in a propellant.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Furoxan NO donor-type statin derivative and preparation method thereof
ActiveCN110128368AGood treatment effectAvoid the problem of mismatching mechanism of actionOrganic chemistryCardiovascular disorderChemical synthesisCoumaric acid
The invention discloses a furoxan NO donor-type statin derivative and a preparation method thereof, and belongs to the technical field of medicinal chemical synthesis. The derivative has the structural formula represented by the following formula: wherein R is an alkyl, R1 is a statin residual group, and R2 is an aryl or an aryl sulfonyl. A statin drug is selected to "hybridize" with a NO donor, both NO and the statin drug have good therapeutic effects on atherosclerosis, and the problem of the mismatch of action mechanisms of NO and the statin drug is effectively avoided. In addition, 4-coumaric acid is selected as a linking group, and thus the therapeutic effect of the drug can be effectively enhanced. The compound can effectively release NO in vitro, and the useful attempt for the development of NO donor anti-atherosclerosis drugs is made.
Owner:CHENGDU UNIV
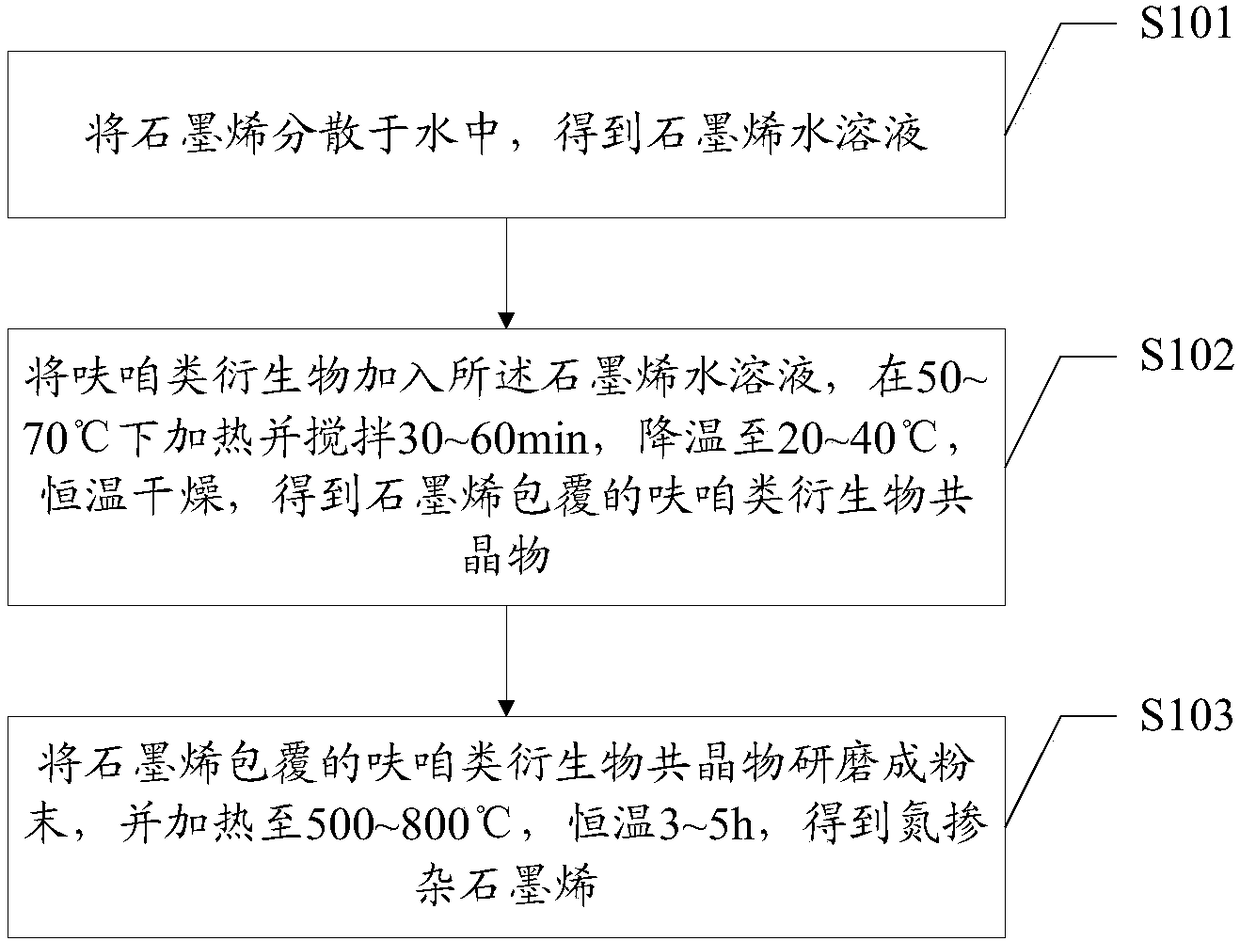
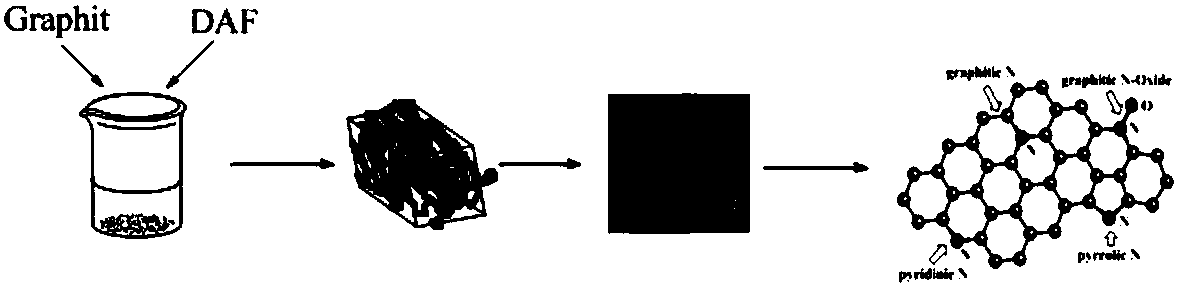
Method for preparing nitrogen-doped graphene from furazan derivatives as nitrogen source
The invention belongs to the technical field of material preparation, and particularly relates to a method for preparing nitrogen-doped graphene from furazan derivatives as a nitrogen source. The method comprises the following steps: dispersing graphene in water to obtain a graphene aqueous solution; adding the furazan derivatives to the graphene aqueous solution, performing heating and stirring at 50-70 DEG C for 30-60 min, performing cooling to 20-40 DEG C, and performing drying at constant temperature to obtain graphene-coated furazan derivative eutectic crystals; grinding the graphene-coated furazan derivative eutectic crystals into powder, and the powder is heated to 500-800 DEG C and kept at the constant temperature for 3-5 h to obtain nitrogen-doped graphene. Graphene in nitrogen-doped graphene uniformly coats the surface of the furazan derivative crystals without using additives such as adhesives and the like, and accordingly, surface performance of the furazan derivative crystals is completely maintained.
Owner:SHENZHEN UNIV
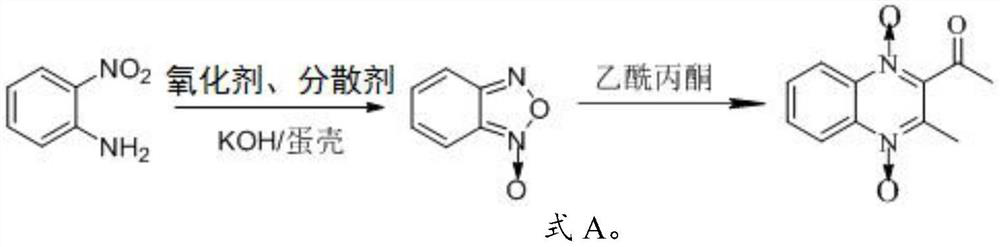
Preparation method of mequindox
PendingCN114044756AReduce releaseProlong the action timeCatalyst carriersOrganic chemistry methodsPtru catalystNitrobenzene
The invention relates to the technical field of veterinary drug synthesis, and provides a preparation method of mequindox. According to the invention, o-nitroaniline and acetylacetone are used as raw materials, mequindox is prepared through an oxidation reaction and a condensation reaction, and the oxidation reaction is carried out under the conditions of an oxidizing agent, a dispersing agent and a catalyst; the dispersing agent can make the solution more uniform, and after the oxidation reaction is completed, benzofurazan does not need to be separated, and the next condensation reaction is directly carried out, so production procedures are reduced, and production efficiency is improved; a KOH / eggshell compound is used as the catalyst, KOH is adsorbed in eggshells and can be slowly released in a reaction process, so the KOH has a slow release effect, and the action time of the catalyst is prolonged; and the waste eggshells are used as a catalyst carrier, so a material source is rich, cost is extremely low, and the catalyst is green and environment-friendly.
Owner:BAYECAO HEALTH IND RES INST (XIAMEN) CO LTD +1
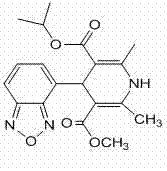
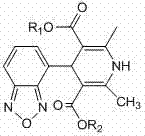
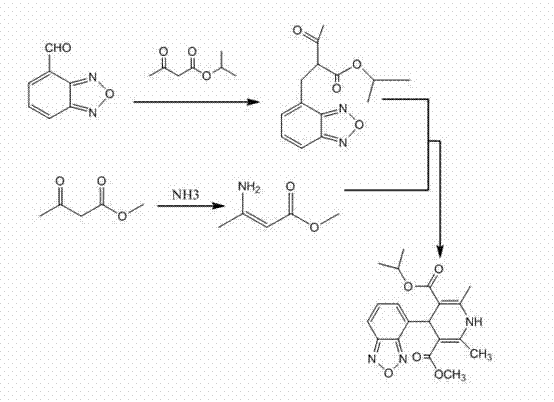
Method for preparing high-purity isradipine
The invention discloses a method for preparing high-purity isradipine. The method includes dehydrating and condensating 4-formaldehyde benzofuroxan in atent solvent to form 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate; subjecting methyl acetoacetate to ammonolysis to form 3-amino crotonic acid methyl ester; subjecting 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate and 3-amino crotonic acid methyl ester to reaction to form isradipine. The method for preparing high-purity isradipine is simple in operation process, convenient to operate, high in yield and capable of preparing high-purity isradipine.
Owner:SICHUAN BAILI PHARM CO LTD
Synthetic method for 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan
The invention provides a synthetic method for 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan. 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan (ANTF) is a low-melting-point (100 DEG C) explosive, is expected to replace TNT to be used in casting explosives, and is also an intermediate for synthesizing other novel explosives. An existing synthetic method of ANTF has the defects of being large in acid use amount, low in synthesis yield, strict in reaction condition and the like. 3,4-bis(3'-aminofurazan-4'-radical) furoxan (BAFF) is adopted as a raw material, 3,4-bis(3'-aminofurazan-4'-radical) furoxan (BAFF) and SnCl2.2H2O are firstly subjected to a reduction reaction to obtain 3,4-bis(aminofurazan-4-raidcal) furazan, and then 3,4-bis(aminofurazan-4-raidcal) furazan and H2O2 are subjected to an oxidizing reaction to obtain the target product 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan. 3,4-bis(aminofurazan-4-raidcal) furazan is adopted as the raw material, H2O2 (20%-30%) is adopted as an oxidizing agent, Na2WO4 is adopted as a catalyst, and 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan is synthesized under the acidic condition through the oxidizing reaction. The synthetic method has the advantages of being easy and convenient to operate, high in safety, controllable in process and the like.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Benzofuroxan derivative and preparation method and application thereof
InactiveCN104876887AHas antitumor activityGood antitumor activityOrganic chemistryAntineoplastic agentsFurazanPharmaceutical drug
The invention relates to the field of a medical technology and discloses a Benzofuroxan derivative and a preparation method and an application thereof. According to the invention, 1,4,5,6,7 sites of a Benzofuroxan structure are subjected to derivatization to obtain the Benzofuroxan derivative. The general molecular formula of the Benzofuroxan derivative is as shown in the specification. The compound provided by the invention has an obvious inhibiting effect on tumor cells and can be used in the preparation of antitumor drugs.
Owner:YANTAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
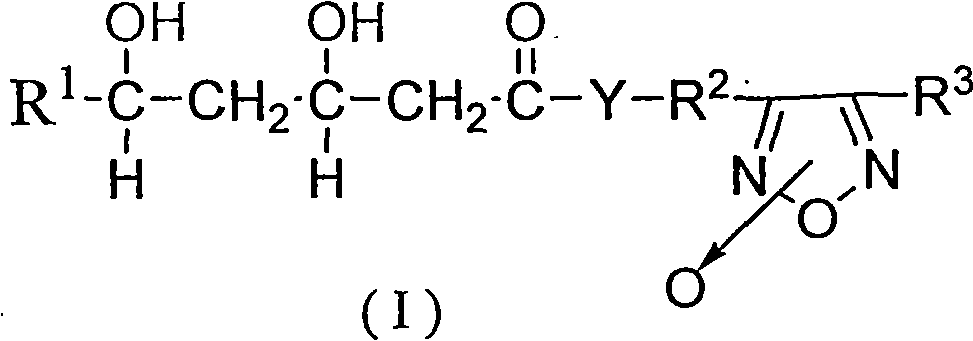
![Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof](https://images-eureka.patsnap.com/patent_img/9439210a-12cf-48f6-9cbf-136ad7c878a4/BSA00000732461400021.PNG)
![Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof](https://images-eureka.patsnap.com/patent_img/9439210a-12cf-48f6-9cbf-136ad7c878a4/BSA00000732461400022.PNG)
![Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof Energy containing ionic salts of 4, 4'-bi [3, 3'-(1-H-5-tetrazolium)] furazan and preparation method thereof](https://images-eureka.patsnap.com/patent_img/9439210a-12cf-48f6-9cbf-136ad7c878a4/BSA00000732461400031.PNG)