Azo-furazan compound and preparing method thereof
An azofurazan and compound technology, which is applied in the field of azofurazan compounds and their preparation, can solve the problems of few and single azofurazan compounds, and achieves the effects of simple preparation, high safety and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 4-aminofurazan-3-amidoxime (AFA)
[0033]
[0034] Preheat 70mL of water to 45°C, add malononitrile (3.20g, 50mmol), and stir at this temperature for 5min. Cool in an ice bath to 0°C-5°C, and add sodium nitrite (3.80 g, 55 mmol). At this temperature, 6 mol / L hydrochloric acid (0.55 mL) was added, and the temperature was gradually raised to 10°C. After 15 minutes, the ice bath was removed, and the stirring reaction was continued for 1.5 hours. Then the reaction system was cooled to about 10°C, and 50% aqueous hydroxylamine solution (9.90 g, 150 mmol) was added in one go. The reaction temperature was gradually raised to 25 °C, and the reaction was continued at this temperature for 1 h. Further reflux reaction for 2h, naturally cooled to room temperature. Under cooling in an ice bath, the temperature of the system was controlled below 5 °C, and the pH was adjusted to 7.0 with 6 mol / L hydrochloric acid (8.0 mL). Filtration, the resulting precipitate was ...
Embodiment 2
[0041] Preparation of 4-amino-3-cyanofurazan
[0042]
[0043] 1.14g (8mmol) of AFA was dissolved in 5ml of acetic acid at 20°C, and 1.78g (7.4mmol) of lead dioxide was added. Stir at room temperature for 5 h, filter, extract with ethyl acetate, neutralize with saturated sodium carbonate solution, dry over anhydrous sodium sulfate, evaporate ethyl acetate to dryness under reduced pressure, add the solid into dichloromethane and stir for 30-60 min, Filtration followed by removal of dichloromethane gave 4-amino-3-cyanofrazan.
[0044] Melting point: 85-87°C.
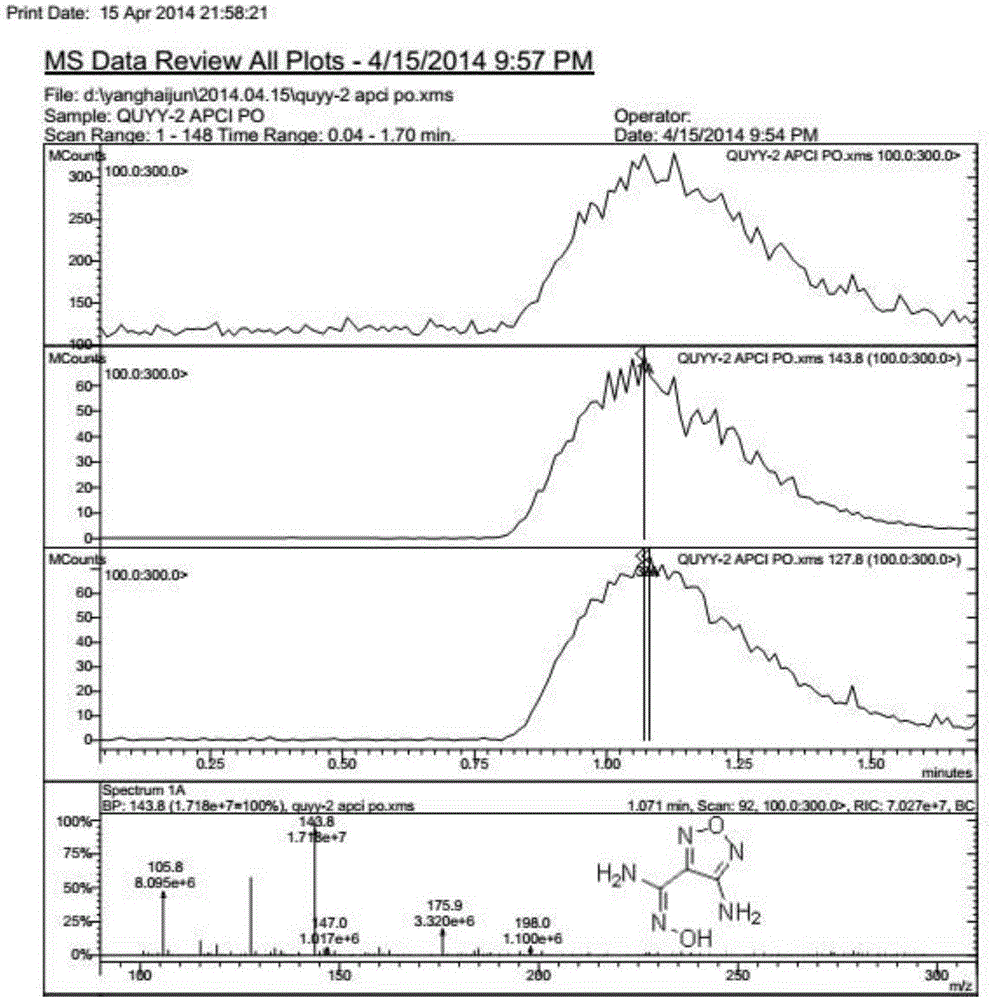
[0045] Mass spectrum: 110 (M+).
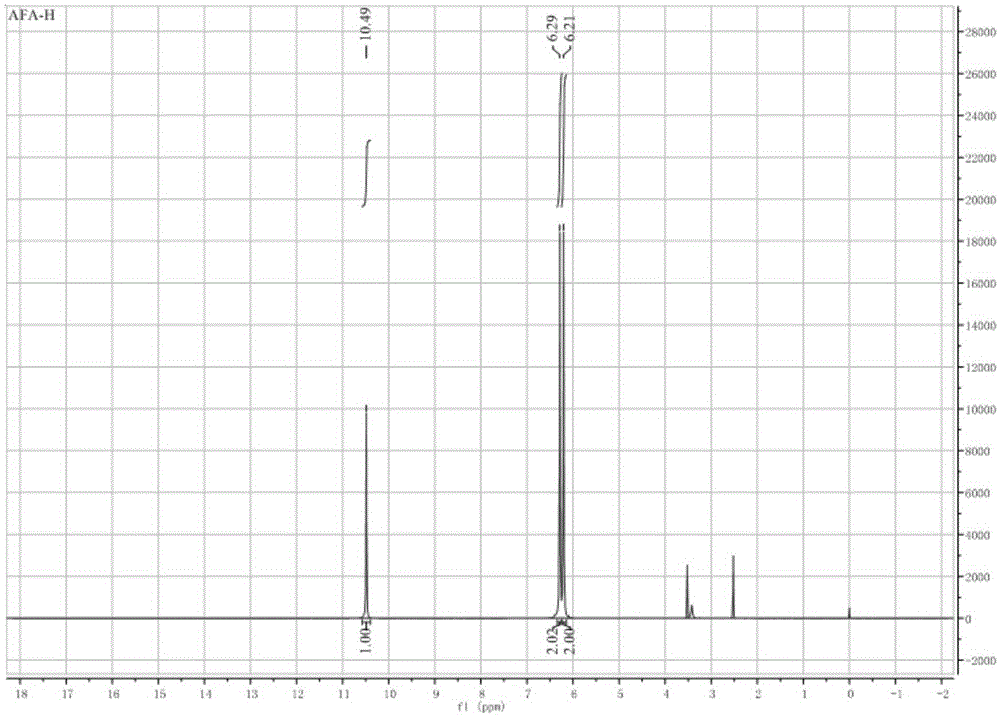
[0046] 1H-NMR: 7.11 (s, 1H).
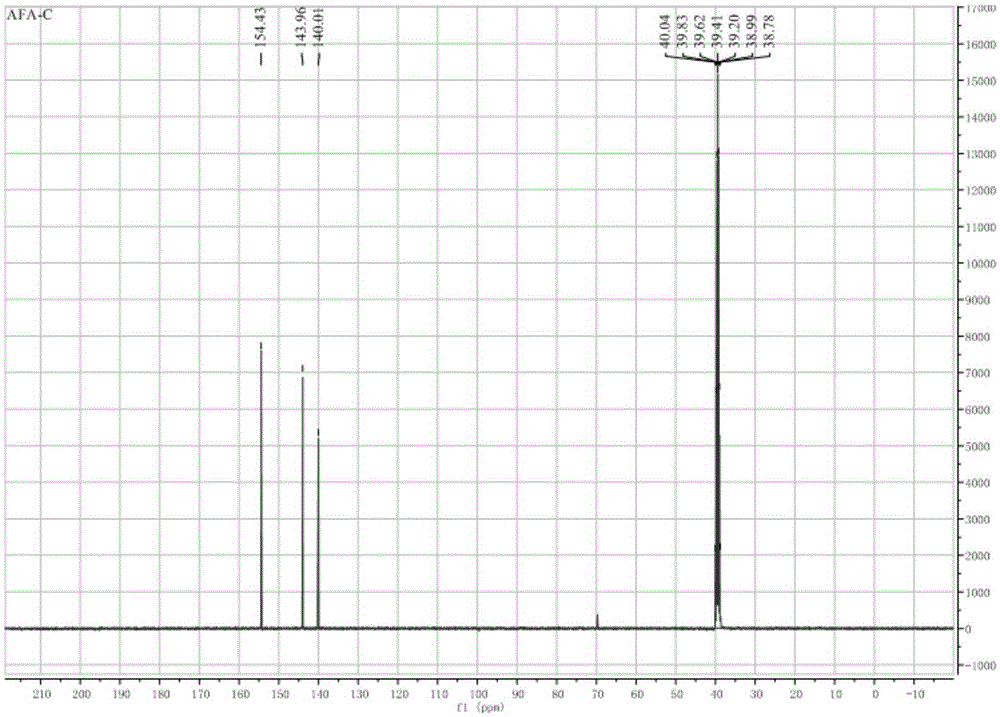
[0047] 13C-NMR: 157.45; 126.47; 108.69.
[0048] Or dissolve 8mmol AFA in 5ml acetic acid at 25°C, add 8mmol lead dioxide and stir at room temperature for 1h, filter, extract with ethyl acetate, neutralize with saturated sodium carbonate solution, dry over anhydrous sodium sulfate, and reduce pressure The ethyl acetate was evaporated to dryness, the s...
Embodiment 3
[0050] Preparation of 3-amino-4-(1,2,3,4-tetrazol-5-yl)furazan
[0051]
[0052] Using the same method as in Example 2, 4-amino-3-cyanofurazan was obtained, and then reacted with hydrazine hydrate at a temperature of 15° C. for 10 h according to a molar ratio of 1:8 to obtain a white solid (4-amino-1,2,5 -oxazole-3-acylhydrazone), white solid and sodium nitrite were reacted for 10h in 20% hydrochloric acid at a molar ratio of 1:2 at a temperature of 35°C to obtain 3-amino-4-(1,2 ,3,4-tetrazol-5-yl)furazan.
[0053] Mass spectrum: 151.8 (M-).
[0054] Or adopt the same method as in Example 2 to obtain 4-amino-3-cyanofurazan, then react with hydrazine hydrate at a temperature of 30° C. for 5 h to obtain a white solid according to a molar ratio of 1:10, and mix the white solid with sodium nitrite in The temperature was 15°C and the reaction was carried out in 15% hydrochloric acid with sufficient concentration for 15 hours according to the molar ratio of 1:5 to obtain 3-amin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com