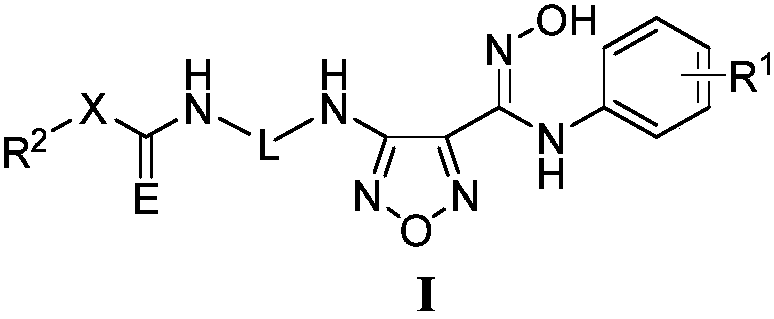
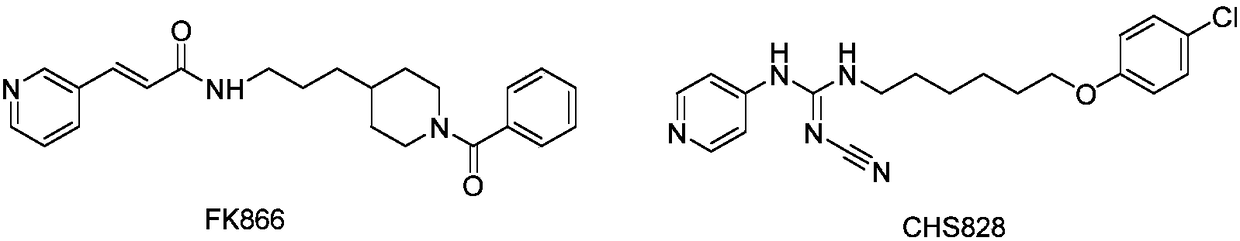
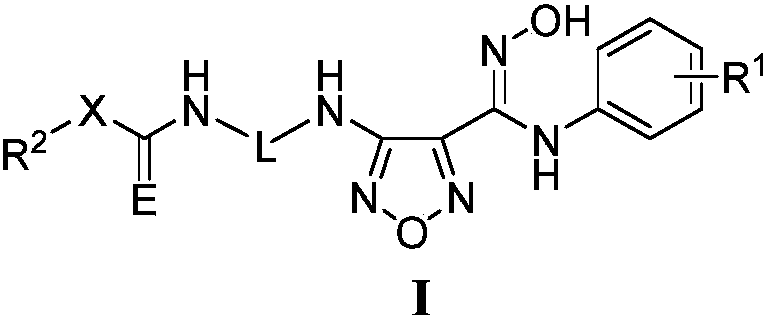
Novel NAMPT and IDO dual inhibitor, and preparation method and medical applications thereof
A dual inhibitor, a new type of technology, applied in the direction of antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problems of high toxicity and side effects, weak curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] (Z)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxymethylimidyl)-1,2,5-oxadiazol-3-yl )amino)ethyl)imidazol[1,2-a]pyridine-6-carboxamide preparation
[0098] (1) Preparation of (Z)-4-amino-N'-hydroxyl-N-(3-bromo-4-fluorophenyl)-1,2,5-oxadiazole-3-methylimidium
[0099]
[0100] At 0°C, acetic acid (5Ml), hydrochloric acid (4N) (5mL), NaNO 2(2.42g, 34.96mmol) in water (5mL) solution, warmed up to room temperature and reacted for 18h, added ethyl acetate (25ml), separated layers, extracted the aqueous phase with ethyl acetate (2*25ml), combined the organic phases, and used saturated Wash with brine (25 mL), dry over anhydrous sodium sulfate, and concentrate to give Intermediate 2 (4.5 g). To a solution of compound 2 (4.5 g) and 3-bromo-4-fluoroaniline (11.8 g, 62.0 mmol) in methanol (20 ml) was added NaHCO under nitrogen protection 3 (7.8g, 93.0mmol) in water (20ml), react at 50°C for 12 hours, TLC detects that the reaction is complete, concentrate, add ethyl acetat...
Embodiment 2
[0111] The difference with Example 1 is: the N-Boc-2-chloroethylamine in the step (2) of the synthetic method step is replaced by N-Boc-3-chloropropylamine, and the obtained (Z)-N-(2 -((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxymethylimidyl)-1,2,5-oxadiazol-3-yl)amino)propyl)imidazole[ 1,2-a] The structural formula of pyridine-6-carboxamide is as follows:
[0112]
[0113] Its H NMR spectrum is: 1 H NMR(400MHz,DMSO):δ11.06(s,1H),9.86(s,1H),8.83(s,1H),8.44(s,1H),7.71(d,J=3.6Hz,1H), 7.65(m,1H),7.46(s,2H),6.93(m,1H),6.76(m,1H),6.64(m,1H),6.05(s,1H),3.35(m,2H),3.05 (m,2H),1.93(m,2H)ppm.
[0114] Its carbon spectrum is: 13 C NMR (125MHz, DMSO): δ165.8, 1634.5, 155.3, 147.2, 145.2, 143.2, 138.4, 134.2, 129.3, 119.7, 117.2, 113.2, 110.6, 42.1, 41.2, 27.8.
[0115] Its mass spectrum is: MS (EI, m / z): 517 (M + +1).
Embodiment 3
[0117] The difference with Example 1 is: the N-Boc-2-chloroethylamine in the step (2) of the synthetic method step is replaced by N-Boc-4-chlorobutylamine, and the obtained (Z)-N-( 2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxymethylimidyl)-1,2,5-oxadiazol-3-yl)amino)butyl)imidazole The structural formula of [1,2-a]pyridine-6-carboxamide is as follows:
[0118]
[0119] Its H NMR spectrum is: 1 H NMR(400MHz,DMSO):δ11.06(s,1H),9.83(s,1H),8.79(s,1H),8.44(s,1H),7.91(d,J=3.5Hz,1H), 7.71(m,1H),7.37(s,2H),6.86(m,1H),6.78(m,1H),6.56(m,1H),6.06(s,1H),3.25(m,2H),3.05 (m,2H),1.50-1.48(m,4H)ppm.
[0120] Its carbon spectrum is: 13 C NMR (125MHz, DMSO): δ167.8, 163.5, 155.2, 153.3, 148.7, 147.2, 145.2, 143.2, 134.4, 133.2, 129.3, 119.2, 118.6, 117.2, 116.7, 114.2, 110.2, 42.1, 39.2, 2
[0121] Its mass spectrum is: MS (EI, m / z): 531 (M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com