Benzofuroxan derivative and preparation method and application thereof
A technology of benzofurazan and derivatives, applied in the field of medicine, can solve the problems of poor long-term curative effect, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
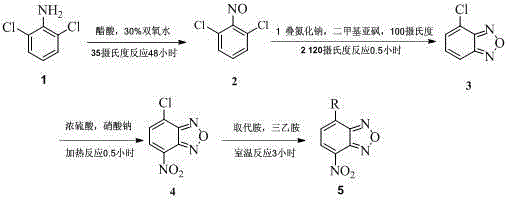
[0079] Example 1: Preparation of class A benzofurazan derivatives 4-amino-7-nitrobenzofurazan
[0080] (1) Preparation of intermediate 2, 6-dichloronitrosobenzene
[0081] In a 100 ml reaction flask, add 2,6-dichloroaniline (1.62 g, 10 mmol), 35 ml of glacial acetic acid and 30% hydrogen peroxide (8.0 ml, 70 mmol), mix well, and react at 35°C for 48 h. After suction filtration and natural drying, 2, 6-dichloronitrosobenzene (1.49 g) was obtained as an off-white flaky solid with a yield of 85.0% and a melting point of 174-175°C.
[0082] (2) Preparation of intermediate 4-chlorobenzofurazan
[0083] In a 100 ml reaction flask, add 2, 6-dichloronitrosobenzene (1.44 g, 8.2 mmol), sodium azide (0.70 g, 11 mmol) and 25 ml of dimethyl sulfoxide, and heat to 100°C , after the bubbling stopped, the temperature was raised to 120°C, and the reaction was continued for 0.5 h. 50 ml of water was added to the reaction system, and a large amount of solid was precipitated. Suction filtrati...
Embodiment 2
[0089] Example 2: Preparation of Class B benzofurazan derivatives 5-amino-6-nitro-1-oxybenzofurazan
[0090] (1) Preparation of intermediate 1, 5-dichloro-2, 4-dinitrobenzene
[0091] In a 100 ml reaction flask, add 15 ml of fuming nitric acid, lower to 0°C, slowly add 2,4-dichloronitrobenzene (4.60 g, 24.3 mmol) dropwise, after the drop is completed, heat up to 50°C, and react for 12 h . The reaction solution was slowly poured into an appropriate amount of crushed ice in batches, and a pale yellow solid was precipitated, which was filtered by suction. The filter cake was washed with water and air-dried to obtain light yellow powder 1, 5-dichloro-2, 4-dinitrobenzene (5.45 g), yield 94.6%, melting point: 100~101°C.
[0092] (2) Preparation of intermediate 1-chloro-5-(4-benzylamino)-2, 4-dinitrobenzene
[0093] In a 50 ml reaction bottle, add 1, 5-dichloro-2, 4-dinitrobenzene (0.59 g, 2.5 mmol), 10 ml absolute ethanol, benzylamine (0.27 g, 2.5 mmol) and triethylamine (0.4 ml...
Embodiment 3
[0099] Example 3: Preparation of Class C Benzofurazan Derivatives 5-Amino-6-Nitrobenzofurazan
[0100] (1) Preparation of 5-(4-benzylamino)-6-nitrobenzofurazan (C 1 )
[0101] Dissolve 5-(4-benzylamino)-6-nitro-1-benzofurazan oxide in an appropriate amount of toluene, add triphenylphosphine, and react at 80°C for 3 hours to generate 5-(4-benzylamino)- 6-Nitrobenzofurazan C 1 (48 mg), yield 29.2%, melting point: 94~95℃. (Spectral data see Table 1 in C 1 )
[0102] Compound C in Table 1 2 The R group is 4-phenylpiperazinyl, and the method is the same as above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com