Lysine-modified benzofuroxan compound, synthetic method, application and recovery method of lysine-modified benzofuroxan compound as well as method of detecting concentration of copper ions
A technology of lysine modification and benzofurazan, applied in chemical instruments and methods, organic chemistry, measuring devices, etc., can solve the problems of time-consuming, complicated preparation process, high cost, etc., and achieve convenient copper ion concentration and sensitivity High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The synthetic method of the compound shown in above structural formula, steps are as follows:
[0035] 1) In an ice-salt bath, stir and mix methanol, thionyl chloride, and lysine for 10-20 minutes; then stir and mix at room temperature for 2-4 hours; then reflux at 70-80°C for 30-40 minutes, and cool to At room temperature, remove methanol, add ether to precipitate solid matter, then cool at 0-6°C for 2-4h, precipitate precipitate, filter, and dry to obtain lysine methyl ester hydrochloride;
[0036] 2) Make a solution of lysine methyl ester hydrochloride and mix it with an acid-binding agent, then drop into a solution of 4-chloro-7-nitrobenzofurazan, fully react at room temperature, and monitor the reaction progress by TLC. After the reaction is completed, filter with suction and remove the solvent to obtain a crude product, dissolve the crude product with dichloromethane, wash with water until the water layer is neutral, remove the acid-binding agent, dry the organic ...
Embodiment 1
[0052] Synthesis of 4-lysine-7-nitrobenzofurazan
[0053] The synthetic route of 4-lysine-7-nitrobenzofurazan is as follows:
[0054]
[0055] The structural formula of 4-lysine-7-nitrobenzofurazan is shown in formula I in the synthesis route, and the specific synthesis steps are as follows:
[0056] 1) Add 5.0 ml of anhydrous methanol to a round bottom flask, cool in an ice-salt bath for 5 min, and add 1.0 ml of thionyl chloride dropwise. After the dropwise addition, 1.0 g (6.85 mmol) of lysine solid was added to the above mixed system at one time, and then the system was first stirred in an ice-salt bath for 10.0 min, then stirred at room temperature for 2 h, and then, at 70 Reflux for 30 min under heating in a water bath at -80 °C, then cool to room temperature;
[0057] After evaporating most of the methanol (60-80vol%) in the solution, 40.0 ml of anhydrous ether was added to the system, and a solid substance was precipitated. Then the system was cooled at 4 °C for...
Embodiment 2
[0064] Detection method of copper ion concentration:
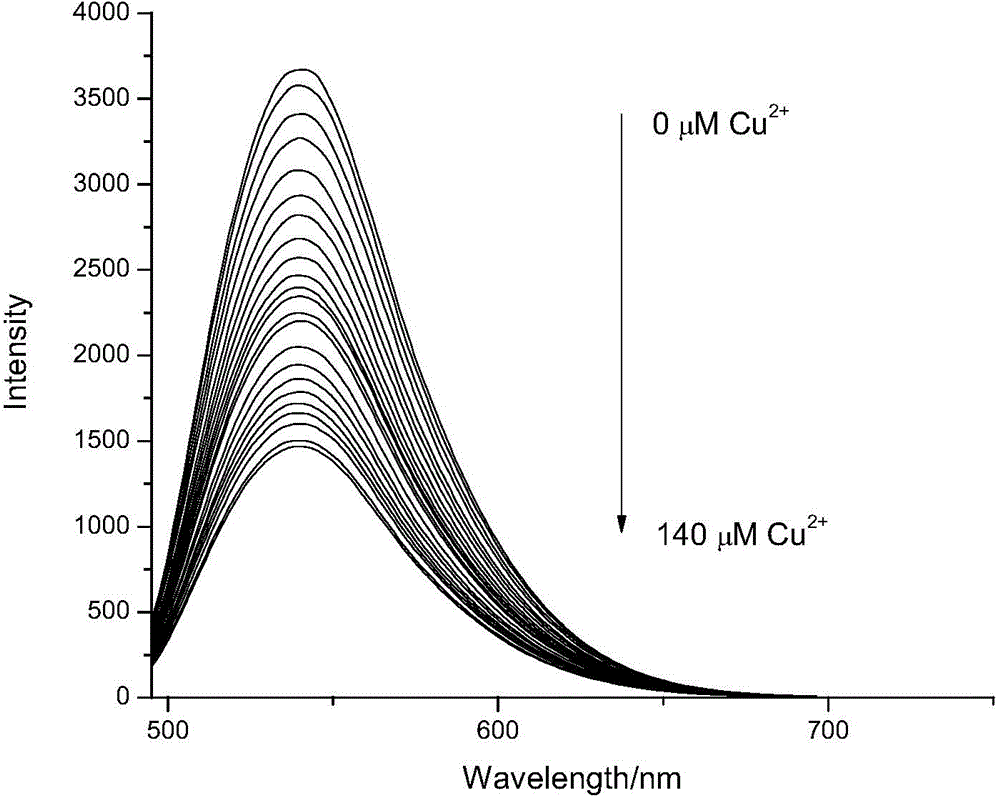
[0065] The preparation concentration is 1.0×10 -5 mol / L 4-lysine-7-nitrobenzofurazan solution, adjust the pH to 5.46, add the concentrated solution of copper ions (concentration is 5 mmol / L) dropwise, so that the concentration of copper ions is 0.0, 1.0, 3.0, 5.0, 8.0, 12, 16, 20, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140 μmol / L, to 470 nm is the excitation wavelength, and their fluorescence spectra are measured, and the fluorescence spectra are shown in figure 1 shown. From figure 1 It can be seen that as the concentration of copper ions increases, the intensity of the fluorescence emission peaks gradually weakens.
[0066] Specific detection:
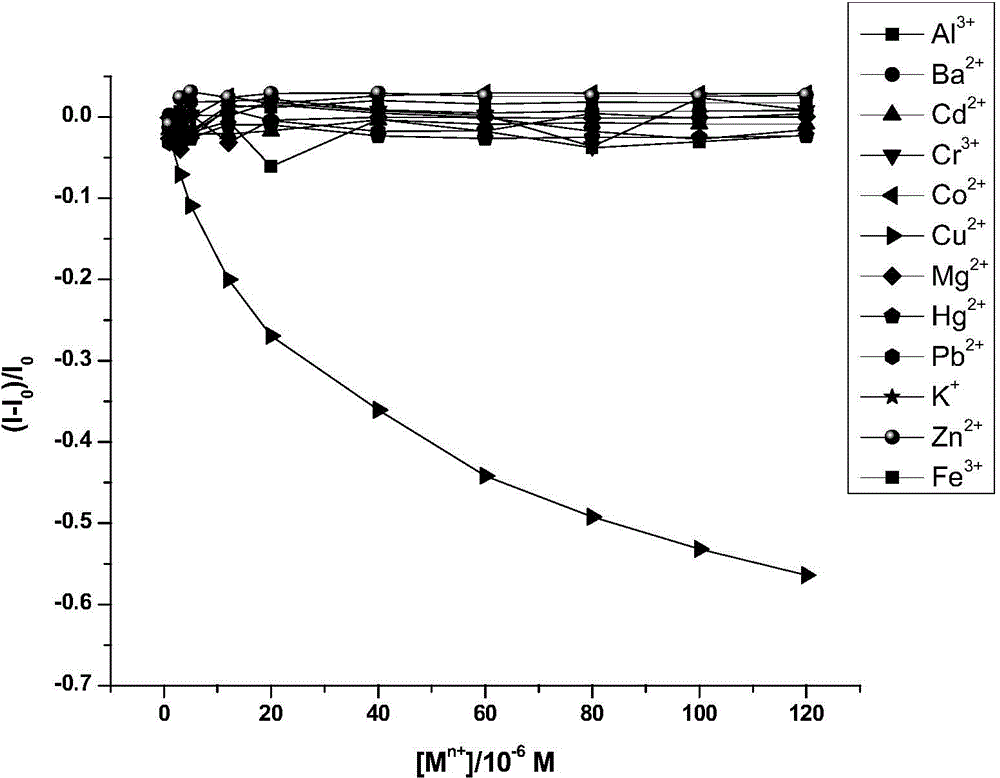
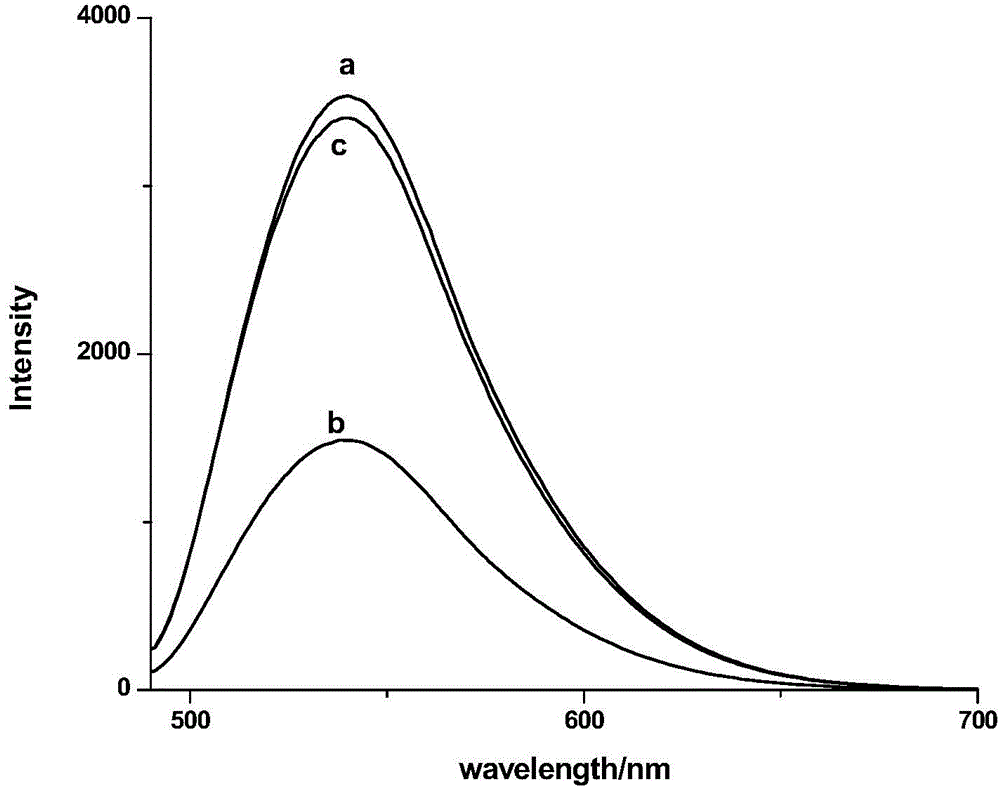
[0067] Detect other metals according to the same steps above, including some heavy metals, alkali metals, alkaline earth metals and transition metals. As a result, these metals do not produce the same phenomenon as copper ions, and the fluorescence inten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com